DOI:
10.1039/D4MD00608A
(Research Article)
RSC Med. Chem., 2025,
16, 221-231
Inhibition of monoamine oxidases by heterocyclic derived conjugated dienones: synthesis and in vitro and in silico investigations†
Received
7th August 2024
, Accepted 11th September 2024
First published on 12th September 2024
Abstract
A total of 18 heterocyclic derived conjugated dienones (CD1–CD18) were evaluated for their potential monoamine oxidase (MAO)-A/-B inhibitory activity. Among the analyzed molecules, CD11 and CD14 showed notable inhibitory potentials against MAO-B, with half-maximal inhibitory concentration (IC50) values of 0.063 ± 0.001 μM and 0.036 ± 0.008 μM, respectively. In contrast, CD1, CD2 and CD3 showed comparable inhibitory activities toward MAO-A, with IC50 values of 3.45 ± 0.07, 3.23 ± 0.24, and 3.15 ± 0.10 μM, respectively. Derivatives of thiophene (CD13–CD17) exhibited selectivity indices greater than 250 for MAO-B. Both lead compounds exhibited similar potencies to safinamide and were more potent than pargyline. According to kinetic analysis, CD11 and CD14 exhibited competitive inhibition of MAO-B activity, with Ki values of 12.67 ± 3.85 nM and 4.5 ± 0.62 nM, respectively. Furthermore, the reversibility test results indicated that the inhibitions were reversible. Molecular docking and molecular dynamics simulation studies can provide insights into the probable binding interactions of CD11 and CD14 with MAO-B. CD11 demonstrated a bipartite contact with Tyr326 and Phe343, whereas CD14 showed contact with Pro102 and Tyr435 via aromatic hydrogen bonds. These results indicated that both compounds have high-affinity binding interactions ( −10.13 and −9.90 kcal mol−1, respectively) at the active site of MAO-B. Furthermore, we used SwissADME to estimate ADME, and both lead compounds demonstrated blood–brain barrier penetration. The study results indicated that all the compounds evaluated demonstrated potent inhibition of MAO-B activity, which was comparable to the efficacy of reference medications. It is necessary to do further investigations on the lead molecules to see whether they may be used to treat different neurodegenerative illnesses.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra, resulting in decreased dopamine (DA) levels in the striatum.1,2 DA deficiency causes motor symptoms in PD, including bradykinesia, rigidity, tremors, and postural instability. Although the exact cause of PD is unknown, several lines of evidence have suggested that factors other than DA depletion, such as dysregulation of the autophagy–lysosomal pathway, oxidative stress, protein aggregation, insufficient support from neurotrophic factors, neuroinflammation, and mitochondrial dysfunction, play crucial roles in PD progression.3–5 The primary objective of contemporary pharmacological approaches to PD is to restore dopaminergic function and alleviate motor dysfunction. Researchers employ a variety of techniques, such as the use of postsynaptic DA receptor direct agonists, inhibition of DA metabolism and reuptake, and DA replacement therapy.6,7 Monoamine oxidase (MAO)-B inhibitors play an important role in dopaminergic modulation because of their sympathomimetic effects on the motor systems of patients with PD.8,9
MAO enzymes are essential for the metabolism of neurotransmitters in the brain. Monoamines, including neurotransmitters such as DA, norepinephrine, and serotonin, are subjected to oxidative deamination.10,11 MAO exists in two isoforms: MAO-A and MAO-B. Although they exhibit structural similarities, their substrate specificities overlap significantly. Both isoforms bind to the substrates dopamine (DA), noradrenaline, adrenaline, and tyramine. In contrast, 2-phenethylamine and benzylamine are selective substrates for MAO-B, whereas serotonin is a substrate for MAO-A.12–15 MAO inhibitors may be used as symptomatic treatments for PD, because they can reverse or at least reduce the decline in DA levels by blocking the MAO-catalyzed metabolism of dopamine in the brain. The MAO-B-selective inhibitors were used to alleviate motor impairments caused by the adverse effects of early non-selective inhibitors. The increase in MAO expression with age, particularly in the heart (6-fold) and neural tissue (4-fold), highlights the importance of MAO-B.16,17 Moreover, gliosis may cause an increase in MAO-B levels in the parkinsonian brain, particularly in the basal ganglia, the area of the brain affected by PD that exhibits higher levels of MAO-B than MAO-A.18,19 Consequently, a variety of central nervous system disorders have been successfully treated with selective MAO isoform-based inhibitors, leading to advancements in medicinal chemistry. MAO-B inhibitors such as safinamide and selegiline are considered adjuvant therapies for PD.20,21 A variety of scaffolds, including chalcones, coumarins, pyrazolines, chromones, isatin derivatives, beta-carbolines, thiazolidinediones, xanthines, thiazoles, base hydrazones, and analogs of Food and Drug Administration (FDA)-approved medications, significantly inhibit MAOs.22–28 Additionally, significant research has been conducted to develop hydrazone/thiosemicarbazone-based MAO inhibitors (MAOIs).
Recently, we synthesized several conjugated dienones using different substituted cinnamaldehydes. Most of these compounds exhibited mild acetylcholinesterase inhibitory activity and displayed potent inhibition of MAO-B within the nanomolar range.29 Heterocyclic group-based conjugated dienones have been designed to further expand upon the structure activity relations (SARs) in this study. Heterocyclic moieties are essential for developing MAO inhibitors, owing to their well-known stability and capacity to engage in hydrophobic, π–π stacking, and hydrogen bonding, which are crucial for inhibiting MAO-A and MAO-B, and consequently aid in the treatment of several neurological and psychiatric conditions.30,31 In this study, we aimed to investigate the SARs of methylenedioxyphenyl, benzofuran, indole, and thiophene heterocyclic rings by synthesizing multiple conjugated dienones by using different substituted cinnamaldehydes. Moreover, the above heterocyclic containing α, β-unsaturated ketones showed more significant MAO-B inhibition than MAO-A.32–36 The study aims to synthesize a new class of heterocyclic conjugated dienones and evaluate their in vitro MAO inhibition, kinetics, and reversibility. The lead molecules were further explored by molecular docking and dynamics simulations.
2. Materials and methods
2.1. Chemicals and reagents
Recombinant MAO-A, MAO-B, kynuramine, benzylamine, safinamide, pargyline, toloxatone, and clorgyline were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anhydrous mono- and dibasic sodium phosphates were purchased from Daejung Chemicals and Metals Co., Ltd. (Siheung, South Korea). A DiaEasy™ dialyzer (6–8 kDa) was purchased from BioVision, Inc., (St. Grove, MA, USA). All other chemicals used in this study were of analytical grade.
2.2. Synthesis
The cinnamaldehyde derivatives (0.01 M) were added using a micropipette to 20 mL of ethanol, and the mixture was stirred before heterocyclic acetophenones (0.01 M) were added. Subsequently, 0.01 M pyrrolidine was added to the mixture and stirred overnight to produce the conjugated dienones (Scheme 1). The products were filtered under suction, carefully cleaned with water, and allowed to dry overnight in a desiccator and crystallize.29
 |
| | Scheme 1 Synthesis of heterocyclic conjugated dienones (CD1–CD18). | |
2.3. Enzyme assays and inhibition studies
MAO-A and MAO-B inhibitory activities were determined using 0.06 mM kynuramine and 0.3 mM benzylamine, respectively, following a standard protocol.37 Absorbance was measured using a standard method.38 The SI of MAO-B was calculated with the following formula: (IC50 of MAO-A)/(IC50 of MAO-B). The inhibitory effects of the test compounds were compared with those of the standard compounds toloxatone and clorgyline for MAO-A and safinamide and pargyline for MAO-B. The IC50 values of the lead compounds were measured at six different concentrations and those of the other effective compounds were measured at three different concentrations. The results are expressed as the mean ± SD of triplicate experiments.
2.4. Enzyme and inhibition kinetics
The enzyme kinetics of the two potent compounds (CD11 and CD14) were determined at five different concentrations of benzylamine (0.0375–0.60 mM) for MAO-B37,39 and three different concentrations of CD11 (10, 20, and 40 nM) and CD14 (5, 10, and 20 nM). Enzyme inhibition patterns and Ki values were determined by comparing LB plots with their respective secondary plots.38
2.5. Reversibility studies
The reversibility of the compounds was evaluated according to standard protocols.37,39 The residual activities AU and AD were analyzed at a concentration of double the IC50 value of CD11 and CD14 after 30 min of preincubation. The recovery of enzyme activity was compared with those of the standard compounds safinamide and pargyline (reversible and irreversible inhibitors, respectively) for MAO-B. Reversibility patterns were determined by comparison with the samples.37,39
2.6. Molecular docking
The crystallographic structure of hMAO-B (PDB code: 2V5Z) and hMAO-A (PDB code: 2X5X) was obtained from the PDB database, RCSB. To preprocess the crystal 3D structure of the protein, Maestro's Protein Preparation Wizard addressed bond ordering, removed superfluous components, and adjusted structural problems such as missing atoms, loops, or side chains.40–42 The centroid box was determined by applying a grid generation method based on the co-crystallized ligand to determine the binding site. The 2D structures of the synthetic CD11 and CD14 compounds were determined, along with low-energy 3D adherents with appropriate bond lengths and angles. For each ligand structure, potential ionization states were generated under the assumption of a physiological pH of 7.2 ± 0.2. The Glide module of the Schrödinger module was used to complete docking; all other parameters were left at their factory default settings.43
2.7. MD simulation
To further investigate the interactions of CD11 and CD14 with MAO-B, molecular simulations were performed by running a 100 ns MD simulation with the aid of the NVIDIA Quadro 6000 graphics processing unit and the Desmond MD simulation program, which had the lowest negative score and highest docking poses.43 For the MD investigations, the box type, thermostat settings, and barometer settings were included, as they were the same as those employed in recent studies and the systems under review. Additionally, short- and long-range interaction calculations were performed. To examine the kinetics of protein–ligand interactions, 100 ns of MD generation was performed, with coordinates saved at 100 ps to yield trajectories of 1000 frames each.43 RMSD and RMSF were computed. Subsequently, stability analysis was performed using the tool for simulated interaction diagrams.
3. Results and discussion
3.1. Synthesis
Pyrrolidine-catalyzed reactions between heterocyclic acetophenones and several substituted cinnamaldehyde derivatives produce heterocyclic conjugated dienones (Scheme 1). All final derivatives were characterized using 1H NMR and 13C NMR spectroscopy (Bruker Advance Neo 500 MHz NMR spectrometer, Billerica, MA, USA), and mass spectrometry (Waters Xevo G2-XS QTOF, Milford, MA, USA). The carbonyl linker of the conjugated dienones was confirmed by a hydrogen peak at 6.5–7.5 ppm with a value of ≈15 Hz associated with the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and a peak in the C13 NMR (range between 188–177 ppm) [ESI†].
O) and a peak in the C13 NMR (range between 188–177 ppm) [ESI†].
3.2. Inhibitory activities on MAO enzymes
The MAO inhibitory activities of the 18 CD compounds were measured following the standard protocol described in the Materials and methods section. Preliminary screening against MAO-A and MAO-B was performed at a concentration of 10 μM of compounds. The results revealed that CD1, CD2, CD3, and CD11 showed <50% residual activity against MAO-A, whereas all compounds except CD10 showed good inhibitory activity toward MAO-B (Table 1). Furthermore, the concentration-dependent inhibitory activities of all compounds against MAO-A and MAO-B were analyzed, and the IC50 values were calculated. The IC50 values ranged from 3.15 to >40 μM for MAO-A and from 0.036 to 17.76 μM toward MAO-B. Among the analyzed compounds, CD11 and CD14 demonstrated the highest inhibitory potential against MAO-B, with IC50 values of 0.063 ± 0.001 and 0.036 ± 0.008 μM, respectively (Table 1 and Fig. 1). In contrast, CD1, CD2, and CD3 exhibited comparable inhibitory potential toward MAO-A with IC50 values of 3.45 ± 0.07, 3.23 ± 0.24, and 3.15 ± 0.10 μM, respectively (Table 1). Our results revealed that these compounds are highly selective for MAO-B. Compounds CD14, CD13, CD17, CD16, CD15, and CD11 exhibited SI values of 376.66, 294.12, 258.38, 248.28, 224.44 and 104.44, respectively. These values indicate that these compounds demonstrate more selectivity toward MAO-B than toward MAO-A (Table 1). When compared with the reference compounds, CD11 and CD14 were more potent than pargyline (0.137 ± 0.01 μM) and less potent than safinamide (0.021 ± 0.002 μM).
Table 1 IC50 values of 18 heterocyclic derived conjugated dienones with MAO-A/-Ba
| Compound |
Residual activity at 10 μM (%) |
IC50 (μM) |
SIb |
| MAO-A |
MAO-B |
MAO-A |
MAO-B |
|
Results are expressed as mean ± standard deviation (SD) of triplicate experiments.
SI values are expressed for MAO-B compared with MAO-A based on their IC50 values.
|
|
CD1
|
31.99 ± 1.88 |
11.59 ± 1.31 |
3.45 ± 0.07 |
2.43 ± 0.70 |
1.43 |
|
CD2
|
12.40 ± 1.62 |
−26.88 ± 0.40 |
3.23 ± 0.24 |
0.101 ± 0.02 |
31.98 |
|
CD3
|
17.44 ± 0.711 |
1.22 ± 0.22 |
3.15 ± 0.10 |
0.91 ± 0.19 |
3.46 |
|
CD4
|
69.9 ± 18.04 |
−19.88 ± 0.45 |
18.84 ± 0.70 |
0.307 ± 0.05 |
61.37 |
|
CD5
|
74.74 ± 12.4 |
31.28 ± 5.81 |
>40 |
6.01 ± 0.64 |
>6.6 |
|
CD6
|
86.79 ± 5.2 |
27.17 ± 4.35 |
>40 |
3.46 ± 0.31 |
>11.56 |
|
CD7
|
96.11 ± 2.77 |
32.38 ± 2.28 |
>40 |
2.99 ± 0.48 |
>13.38 |
|
CD8
|
86.92 ± 0.55 |
−21.5 ± 2.17 |
>40 |
0.230 ± 0.01 |
>173.91 |
|
CD9
|
77.71 ± 2.29 |
27.46 ± 0.76 |
>40 |
5.67 ± 0.28 |
>7.05 |
|
CD10
|
50.66 ± 1.97 |
74.69 ± 13.1 |
12.73 ± 0.34 |
17.76 ± 1.10 |
0.72 |
|
CD11
|
43.57 ± 1.6 |
−17.61 ± 2.28 |
6.58 ± 0.69 |
0.063 ± 0.001 |
104.44 |
|
CD12
|
52.27 ± 1.31 |
47.59 ± 7.47 |
12.98 ± 2.0 |
11.51 ± 0.38 |
1.13 |
|
CD13
|
68.77 ± 2.02 |
−5.27 ± 0.40 |
>40 |
0.136 ± 0.008 |
>294.12 |
|
CD14
|
51.81 ± 2.92 |
−5.2 ± 1.7 |
13.56 ± 0.65 |
0.036 ± 0.008 |
376.66 |
|
CD15
|
65.60 ± 3.87 |
−16.45 ± 0.29 |
22.22 ± 3.92 |
0.095 ± 0.006 |
224.44 |
|
CD16
|
71.3 ± 1.33 |
−27.33 ± 2.0 |
24.58 ± 0.95 |
0.099 ± 0.02 |
248.28 |
|
CD17
|
67.07 ± 6.73 |
−11.91 ± 0.33 |
25.63 ± 11.03 |
0.40 ± 0.07 |
258.38 |
|
CD18
|
59.64 ± 8.73 |
−9.3 ± 2.55 |
19.82 ± 5.08 |
0.76 ± 0.02 |
26.07 |
| Safinamide |
— |
— |
— |
0.021 ± 0.002 |
— |
| Pargyline |
— |
— |
— |
0.137 ± 0.01 |
— |
| Toloxatone |
— |
— |
1.77 ± 0.22 |
— |
— |
| Clorgyline |
— |
— |
0.0077 ± 0.0006 |
— |
— |
 |
| | Fig. 1 Concentration-dependent inhibition of MAO-B by CD11 (A) and CD14 (B). The results are expressed as mean ± SD of triplicate experiments. | |
3.3. Structure–activity relationship (SAR)
The 18 synthetic heterocyclic conjugated dienones had four different scaffolds: methylenedioxyphenyl (CD1–CD3), benzofuran (CD4–CD8), indole (CD9–CD11), and thiophene (CD12–CD18) in the A ring and different conjugated dienones in the B ring. CD1, CD2, and CD3 showed comparable inhibitory activities against MAO-A; however, the –OCH3 (CD3) or –Br (CD2) substitution in the B ring showed a slightly higher inhibitory potential than the –NO2 substitution in CD1. The addition of benzofuran (CD4–CD7) led to the inhibition of MAO-B in the micromolar range with a relatively good SI. However, the addition of –NO2, –Br, and –OCH3 groups to the B ring significantly decreased MAO-B inhibition. This trend was also observed in benzofuran-based dienones, in which the placement of the fluorine atoms resulted in good MAO-B inhibition. The bioisosteric replacement of benzofuran (CD7) with indole (CD11) drastically increased (approximately 50 times) MAO-B inhibition.
In contrast, CD11 and CD14 were the most potent inhibitors of MAO-B. Upon examining their structural differences, it was observed that both compounds had similar substitutions in the conjugated dienones at the B ring, but they differed in the A ring. CD11 has an indole scaffold, whereas CD14 contains a thiophene scaffold. Our results clearly indicate that the thiophene scaffold with the –OCH3 (CD14) group substitution was the most potent inhibitor of MAO-B and increased the inhibition potential by 1.75-fold compared to the indole scaffold with the same substitution (CD11). When the same indole scaffolds were compared, the –OCH3 (CD11) group substantially increased the inhibitory potential against MAO-B, and was 280-fold higher than that of the Br (CD10) group. When comparing the activities with similar scaffolds and different conjugated substitutions in the B ring, the Br-substituted thiophene backbone (CD15–CD18) was observed to be more effective and highly selective against MAO-B than MAO-A; however, the inhibitory activities decreased in the following order: substitution of –H (CD15), –F (CD16), –Br (CD17), and –OCH3 (CD18) groups. Comparable inhibition was observed in conjugated dienones based on thiophene when the MAO-B inhibitory profile of the reversibly selective MAO-B inhibitor safinamide was compared. Notably, heterocycles based on thiophene demonstrated 2–2.5 times greater potency than pargyline, an irreversible MAO-B inhibitor approved by the FDA. A pictorial representation of the SAR of the heterocyclic conjugated dienone derivatives is shown in Fig. 2.
 |
| | Fig. 2 SAR of the heterocyclic conjugated dienone derivatives. | |
3.4. Kinetics of MAO-B inhibition
The kinetics of the leading compounds, CD11 and CD14, against MAO-B were evaluated to determine the type of inhibition. Three different concentrations of the inhibitor (0.5, 1.0, and 2.0 × IC50 values) against five different concentrations of benzylamine were used as substrates for this experiment. Lineweaver–Burk (LB) plots of the inhibitors revealed that CD11 and CD14 were competitive MAO-B inhibitors (Fig. 3). Furthermore, the secondary plot showed that the inhibition constant (Ki) values were of 12.67 ± 3.85 and 4.5 ± 0.62 nM, respectively, for CD11 and CD14 inhibitors (Fig. 3).
 |
| | Fig. 3 Lineweaver–Burk (LB) plots for MAO-B inhibition of CD11 (A) and CD14 (C), and the respective secondary plots (i.e., slope vs. inhibitor concentration) of CD11 (B) and CD14 (D). The experiments were conducted at five different concentrations of benzylamine and three different concentrations of CD11 or CD14. The results are expressed as the mean ± SD of triplicate experiments. | |
3.5. Reversibility studies of MAO-B inhibition
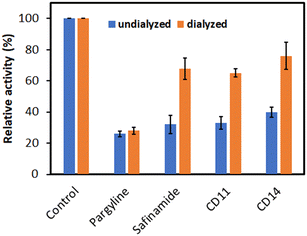
The reversibility of MAO-B inhibition by CD11 or CD14 was evaluated using the dialysis method described in the Materials and methods section. The concentrations of the inhibitors (CD11 and CD14) were approximately double their IC50 values. The enzyme activity recovery was determined by comparing the relative undialyzed (AU) and dialyzed (AD) activities. The inhibition of MAO-B by CD11 recovered from 33.00% (AU) to 65.11% (AD), whereas CD14 recovered from 39.74% (AU) to 76.07% (AD). The results obtained for the compound recoveries were similar to that of safinamide, a reversible inhibitor, from 32.00% to 67.87%, which was significantly different from the compound recovery of pargyline, which is an irreversible inhibitor (from 25.93% to 27.96%), after dialysis (as shown in Fig. 4). These results reveal that both CD11 and CD14 are reversible inhibitors of MAO-B.
 |
| | Fig. 4 Recovery of MAO-B inhibition by CD11 and CD14 using dialysis. The concentrations of CD11 and CD14 used were approximately double the IC50 values. After 30 min of preincubation, the mixtures were dialyzed for 6 h with buffer change at the 3 h interval. The results are expressed as mean ± SD of triplicate experiments. | |
3.6. Molecular docking
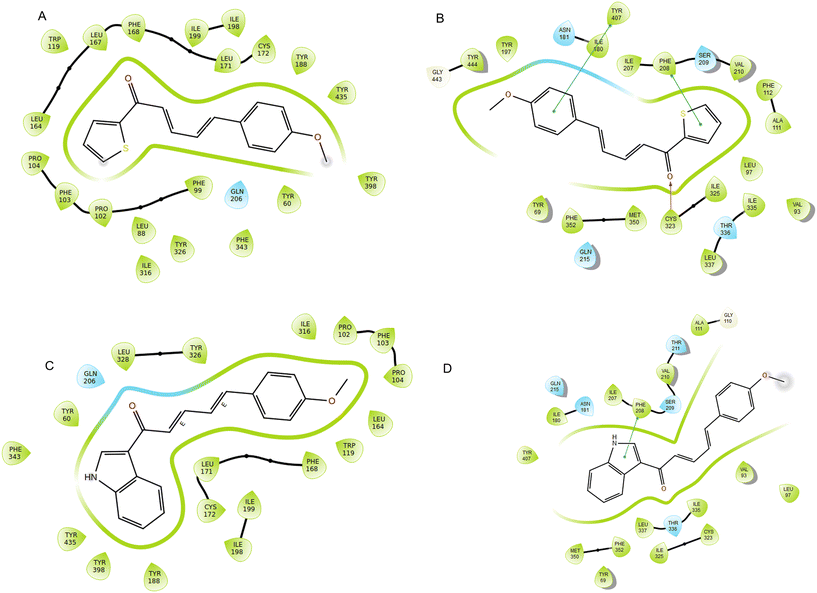
Molecular docking aims to predict the binding affinity and interaction between two molecules, which is an important task in protein engineering, drug discovery, and comprehension of biochemical pathways. A docking study was performed to investigate the interaction of CD11 and CD14 with hMAO-A and hMAO-B co-crystallized with the native ligands using the Glide module. For hMAO-A, the docking scores (XP mode) for CD11 and CD14 were −4.14 and −2.21 kcal mol−1, respectively. The residue Cys323 engaged in hydrogen bonding with the carbonyl group of CD14. Through π–π stacking, Tyr407 and Phe208 were connected to phenyl and thiophene rings, respectively. In the case of CD11, significant interaction was observed with the indole ring of Phe208 through π–π stacking (Fig. 5B and D). The docking scores (XP modes) of the lead compounds CD11 and CD14 were −10.13 kcal mol−1 and −9.90 kcal mol−1, respectively for hMAO-B. These scores were similar to that of native safinamide ( −11.40 kcal mol−1). Hydrophobic interactions with Tyr60, Leu88, Phe99, Phe103, Pro102, Pro104, Trp119, Leu164, Leu167, Phe168, Leu171, Cys172, Tyr188, Gln206, Tyr435, Ile316, Tyr398, Leu164, Tyr326, Leu328, Ile198, and Ile199 were obtained for the two lead compounds as shown in the 2D interaction diagram (Fig. 5A and C). The ketone group of CD11 forms a bipartite contact with Tyr326 and Phe343 through hydrogen bonding and aromatic interactions. Similarly, the methoxy-attached phenyl ring of CD14 engaged in an aromatic hydrogen bond with Tyr435 as well as with the thiophene ring and Pro102. Upon comparing the docking contacts of the two lead compounds, it was observed that the indole moiety in CD11 shared similarities with that in flavin adenine dinucleotide (FAD), whereas the methoxy chain of CD14 was like that of FAD (Fig. S1†). Similar patterns were observed in the biological activity (IC50) and docking scores for MAOs, i.e., both lead compounds exhibited a higher binding affinity towards MAO-B than MAO-A. The study results indicate that the orientation of the binding pocket was altered when the thiophene rings were modified with indole.
 |
| | Fig. 5 2D ligand interactions of CD14 and CD11 in the binding pocket of 2V5Z (A and C) and 2Z5X (B and D). | |
3.7. Molecular dynamics simulation
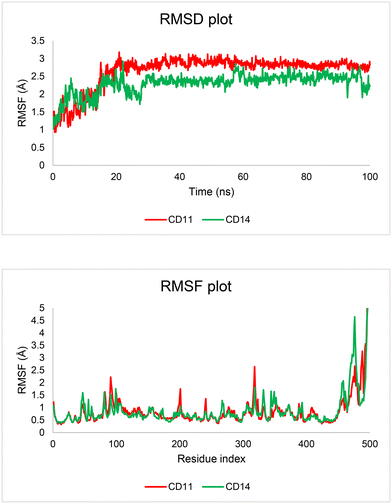
Molecular dynamics (MD) is a powerful tool for understanding and simulating the behavior of atomic-level molecular systems. MD simulations have a significant impact on materials science, biology, and chemistry because they provide deep insight into the dynamics and interactions of atoms and molecules. By simulating the dynamic behavior of a protein–ligand complex with near accuracy or realism, MD simulations have been utilized in drug discovery research to swiftly and effectively explore the energetic aspects of protein and ligand interactions. In this study, MD simulations were used to mimic CD11 and CD14 at the binding site of MAO-B in a biological context. Using MD trajectories, protein–ligand interactions, root-mean-square deviation (RMSD), and root-mean-square fluctuation (RMSF) were computed. In MD simulations, RMSD is an essential statistic for evaluating the structural stability and similarity. It aids researchers in comprehending the dynamic behavior of biomolecules and how they react to different circumstances by offering a quantifiable measure of deviation. RMSD provides important insights into the molecular mechanisms involved including conformational changes, ligand binding, and protein folding. Over the simulation period, a stable ligand–protein combination was shown by the RMSD plot (Fig. 6), where the RMSD values for the protein C-alpha atoms in the complexes with CD11 and CD14 ranged from 0.93 to 3.18 Å and 1.02 to 2.92 Å, respectively. For CD11 and CD14, the average RMSD values were 2.65 Å and 2.23 Å, respectively. The highest protein RMSD was obtained at 20 ns, at which point the RMSD values stabilized at 3.18 Å and 2.92 Å. Regarding the protein and its binding site, the overall RMSD values demonstrated that CD14 and CD11 were notably more stable. Another crucial metric for evaluating the dynamic behavior and flexibility of certain atoms, residues, or areas inside a molecule, usually a protein or nucleic acid in MD simulations, is RMSF. In general, RMSD measures the structural deviation from the reference, whereas RMSF focuses on atom-by-atom fluctuations around the average positions during the simulation. The N- and C-terminal residues exhibited relatively fewer and generally higher fluctuations, respectively, as shown in the RMSF plot (Fig. 6). MAO-B has 22 and 17 amino acid residues in contact with CD11 and CD14, respectively (Table 2). Protein C-alpha atoms in complexes with CD11 and CD14 have RMSF values ranging from 0.42 to 1.63 Å and 0.44 to 0.79 Å, respectively. A deeper understanding of the binding patterns will be made possible by a more precise representation of the physiological ambient conditions provided by MD simulations. According to the trajectory analysis and the overall results of the MD simulation, these molecules were effective in inhibiting MAO-B.
 |
| | Fig. 6 MD simulation analysis of CD11 and CD14 complexes with MAO-B proteins. RMSD and RMSF of various amino acids. | |
Table 2 Amino acid residues contacting with the ligand and their RMSF values
| Code |
Contacting amino acid and RMSF (Å) |
|
CD11
|
Tyr60 (0.496), Glu84 (1.631), Pro102(1.588), Phe103(1.196), Pro104 (0.971), His115 (0.915), Trp119 (0.884), Leu164 (0.816), Leu167 (0.759), Phe168 (0.698), Leu171 (0.729), Ile199 (0.912), Ser200 (1.068), Thr201 (1.079), Gln206 (0.634), Glu207 (0.717), Ile316 (0.758), Tyr326 (0.753), Leu328 (0.543), Phe343 (0.551), Tyr398 (0.422), and Tyr435 (0.442) |
|
CD14
|
Leu88 (0.596), Phe99 (0.772), Pro104 (0.792), Trp119 (0.701), Leu164 (0.626), Phe168 (0.558), Leu171 (0.781), Tyr188 (0.447), Ile198 (0.69), Ile199 (0.795), Ile316 (0.733), Tyr326 (0.643), Phe343 (0.528), Tyr398 (0.547), Ser433 (0.462), Gly434 (0.546), and Tyr435 (0.529) |
Swiss ADME prediction.
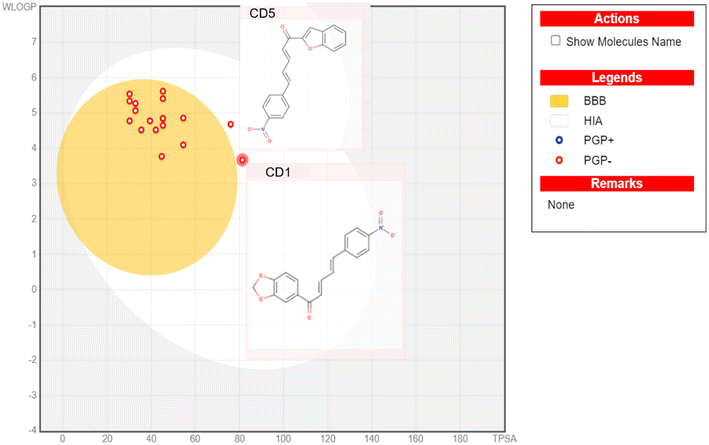
We computed ADME attribute predictions for our synthesized compounds. Our synthesized compounds did not violate the well-known Lipinski's rule, Ghose, Veber, or Egan rules.44 Its placement in the yellow area of the boiled egg model suggests that it may have an opportunity to cross the blood–brain barrier (BBB).45 The brain or intestinal estimated permeation method (BOILED-Egg) is a proposed precise predictive model. It works by figuring out how polar and lipophilic tiny molecules are. The same two physicochemical descriptors are used to provide concurrent predictions for brain and gut penetration, which are then directly translated into molecular design because of the model's quickness, precision, conceptual simplicity, and easily understandable graphical output. With the possible exception of compounds CD1 and CD5 (Table 3), which did not exhibit BBB permeability due to the presence of a strong deactivate group (nitro), all other compounds showed BBB permeant property (Fig. 7).
Table 3 Heterocyclic conjugated dienones for predicted GI absorption, BBB permeability, and bioavailability score
| Compound code |
GI absorption |
BBB permeant |
Bioavailability score |
|
CD1
|
High |
No |
0.55 |
|
CD2
|
High |
Yes |
0.55 |
|
CD3
|
High |
Yes |
0.55 |
|
CD4
|
High |
Yes |
0.55 |
|
CD5
|
High |
No |
0.55 |
|
CD6
|
High |
Yes |
0.55 |
|
CD7
|
High |
Yes |
0.55 |
|
CD8
|
High |
Yes |
0.55 |
|
CD9
|
High |
Yes |
0.55 |
|
CD10
|
High |
Yes |
0.55 |
|
CD11
|
High |
Yes |
0.55 |
|
CD12
|
High |
Yes |
0.55 |
|
CD13
|
High |
Yes |
0.55 |
|
CD14
|
High |
Yes |
0.55 |
|
CD15
|
High |
Yes |
0.55 |
|
CD16
|
High |
Yes |
0.55 |
|
CD17
|
High |
Yes |
0.55 |
|
CD18
|
High |
Yes |
0.55 |
 |
| | Fig. 7 BOILED-Egg plot of the heterocyclic conjugated dienones generated from SWISS ADME. | |
4. Conclusion
In this study, we assessed the inhibition of human MAOs by synthesizing and examining eighteen heterocyclic conjugated dienones, both in vitro and in silico. Notably, all derivatives exhibited strong selective MAO-B inhibitory activities in the micromolar to nanomolar range. The most effective inhibitory activity against MAO-B was exhibited by CD11 and CD14 with IC50 values of 63 and 34 nM, respectively. Studies on the enzyme kinetics and reversibility of CD11 and CD14 have demonstrated that they are competitive and reversible inhibitors of MAO-B. MD and docking experiments have shed new light on the binding mechanism of MAO-B inhibitors. Further, we found our lead molecules were BBB permeable by using the SwissADME prediction tool. Consequently, our results imply that CD11 and CD14 may be used therapeutically to treat various neurodegenerative conditions including PD.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
Conceptualization: B. M. and H. K.; chemical synthesis: S. K. and B. M. biological evaluation: B. P. P. and H. K.; computational studies: S. K. and M. A. A.; writing – original draft preparation: S. K., B. P. P. and M. A. A.; writing – review and editing: M. M. G., B. M., R. B. B. and H. K.; funding acquisition: M. M. G. supervision: B. M. and H. K. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to AlMaarefa University for their support.
References
- E. Guatteo, N. Berretta, V. Monda, A. Ledonne and N. B. Mercuri, Pathophysiological Features of Nigral Dopaminergic Neurons in Animal Models of Parkinson's Disease, Int. J. Mol. Sci., 2022, 23(9), 4508, DOI:10.3390/ijms23094508.
- P. Feraco, C. Gagliardo, G. L. Tona, E. Bruno, C. D'angelo, M. Marrale, A. D. Poggio, M. C. Malaguti, L. Geraci, R. Baschi, B. Petralia, M. Midiri and R. Monastero, Imaging of Substantia Nigra in Parkinson's Disease: A Narrative Review, Brain Sci., 2021, 11(6), 769, DOI:10.3390/brainsci11060769.
- I. Hinarejos, C. Machuca-Arellano, P. Sancho and C. Espinós, Mitochondrial Dysfunction, Oxidative Stress and Neuroinflammation in Neurodegeneration with Brain Iron Accumulation (NBIA), Antioxidants, 2020, 9(10), 1020, DOI:10.3390/antiox9101020.
- S. R. Bonam, C. Tranchant and S. Muller, Autophagy-Lysosomal Pathway as Potential Therapeutic Target in Parkinson's Disease, Cell, 2021, 10(12), 3547, DOI:10.3390/cells10123547.
- N. A. Gouda, A. Elkamhawy and J. Cho, Emerging Therapeutic Strategies for Parkinson's Disease and Future Prospects: A 2021 Update, Biomedicines, 2022, 10(2), 371, DOI:10.3390/biomedicines10020371.
- T. Müller, Experimental Dopamine Reuptake Inhibitors in Parkinson's Disease: A Review of the Evidence, J. Exp. Pharmacol., 2021, 13, 397–408, DOI:10.2147/JEP.S267032.
- S. J. Kish, I. Boileau, R. C. Callaghan and J. Tong, Brain dopamine neurone 'damage': methamphetamine users vs. Parkinson's disease - a critical assessment of the evidence, J. Exp. Pharmacol., 2017, 45(1), 58–66, DOI:10.1111/ejn.13363.
- Y. Y. Tan, P. Jenner and S. D. Chen, Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson's Disease: Past, Present, and Future, J. Parkinson's Dis., 2022, 12(2), 477–493, DOI:10.3233/JPD-212976 , PMID: 34957948.
- W. H. Jost, A critical appraisal of MAO-B inhibitors in the treatment of Parkinson's disease, J. Neural Transm., 2022, 129(5–6), 723–736, DOI:10.1007/s00702-022-02465-w , Epub 2022 Feb 2. PMID: 35107654.
- D. N. Jones and M. A. Raghanti, The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders, J. Chem. Neuroanat., 2021, 101957, DOI:10.1016/j.jchemneu.2021.101957.
- M. Naoi, W. Maruyama and M. S. Nagai, Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies, Int. J. Mol. Sci., 2022, 23(19), 11059, DOI:10.3390/ijms231911059.
- S. Manzoor and N. Hoda, A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer's disease agents: A review, Eur. J. Med. Chem., 2020, 206, 112787, DOI:10.1016/j.ejmech.2020.112787.
- P. Guglielmi, S. Carradori, I. D'Agostino, C. Campestre and J. P. Petzer, An updated patent review on monoamine oxidase (MAO) inhibitors, Expert Opin. Ther. Pat., 2022, 32(8), 849–883, DOI:10.1080/13543776.2022.2083501.
- M. A. Abdelgawad, J. M. Oh, D. G. Parambi, S. Kumar, A. Musa, M. M. Ghoneim, A. A. Nayl, A. H. El-Ghorab, I. Ahmad, H. Patel and H. Kim, Development of bromo-and fluoro-based α, β-unsaturated ketones as highly potent MAO-B inhibitors for the treatment of Parkinson's disease, J. Mol. Struct., 2022, 1266, 133545 CAS.
- C. Pierre, J. Callebert, J. M. Launay, J. Leclercq and S. Rétaux, The enzymatic and neurochemical outcomes of a mutation in Mexican cavefish MAO reveal teleost-specific aspects of brain monoamine homeostasis, J. Exp. Biol., 2023, 226(14), jeb245448, DOI:10.1242/jeb.245448.
- S. Ramesh and A. S. P. M. Arachchige, Depletion of dopamine in Parkinson's disease and relevant therapeutic options: A review of the literature, AIMS Neurosci., 2023, 10(3), 200–231, DOI:10.3934/Neuroscience.2023017.
- A. Mannan, T. G. Singh, V. Singh, N. Garg, A. Kaur and M. Singh, Insights into the Mechanism of the Therapeutic Potential of Herbal Monoamine Oxidase Inhibitors in Neurological Diseases, Curr. Drug Targets, 2022, 23(3), 286–310, DOI:10.2174/1389450122666210707120256.
- P. Duarte, P. Michalska, E. Crisman, A. Cuadrado and R. León, Novel Series of Dual NRF2 Inducers and Selective MAO-B Inhibitors for the Treatment of Parkinson's Disease, Antioxidants, 2022, 11(2), 247, DOI:10.3390/antiox11020247.
- G. Prunell and S. Olivera-Bravo, A Focus on Astrocyte Contribution to Parkinson's Disease Etiology, Biomolecules, 2022, 12(12), 1745, DOI:10.3390/biom12121745.
- T. Behl, D. Kaur, A. Sehgal, S. Singh, N. Sharma, G. Zengin, F. L. Andronie-Cioara, M. M. Toma, S. Bungau and A. G. Bumbu, Role of Monoamine Oxidase Activity in Alzheimer's Disease: An Insight into the Therapeutic Potential of Inhibitors, Molecules, 2021, 26(12), 3724, DOI:10.3390/molecules26123724.
- D. Osmaniye, B. Kurban, B. N. Sağlık, S. Levent, Y. Özkay and Z. A. Kaplancıklı, Novel Thiosemicarbazone Derivatives: In Vitro and In Silico Evaluation as Potential MAO-B Inhibitors, Molecules, 2021, 26(21), 6640, DOI:10.3390/molecules26216640.
- S. Carradori, D. Secci and J. P. Petzer, MAO inhibitors and their wider applications: a patent review, Expert Opin. Ther. Pat., 2018, 28(3), 211–226 CrossRef CAS PubMed.
- S. T. Sudevan, T. M. Rangarajan, A. G. Al-Sehemi, A. S. Nair, V. P. Koyiparambath and B. Mathew, Revealing the role of the benzyloxy pharmacophore in the design of a new class of monoamine oxidase-B inhibitors, Arch. Pharm., 2022, 355(8), 2200084 CrossRef CAS.
- B. Mathew, J. Suresh, S. Anbazhagan and G. E. Mathew, Pyrazoline: A promising scaffold for the inhibition of monoamine oxidase, Cent. Nerv. Syst. Agents Med. Chem., 2013, 13(3), 195–206 CrossRef CAS PubMed.
- B. Mathew, A. Haridas, J. Suresh, G. E. Mathew, G. Uçar and V. Jayaprakash, Monoamine oxidase inhibitory action of chalcones: A mini review, Cent. Nerv. Syst. Agents Med. Chem., 2016, 16(2), 120–136 CrossRef CAS.
- V. P. Koyiparambath, K. P. Rajappan, T. M. Rangarajan, A. G. Al-Sehemi, M. Pannipara, V. Bhaskar, A. S. Nair, S. T. Sudevan, S. Kumar and B. Mathew, Deciphering the detailed structure–activity relationship of coumarins as Monoamine oxidase enzyme inhibitors—An updated review, Chem. Biol. Drug Des., 2021, 98(4), 655–673 CrossRef CAS PubMed.
- F. Benny, S. Kumar, J. Jayan, M. A. Abdelgawad, M. M. Ghoneim, A. Kumar, A. Manoharan, R. Susan, S. T. Sudevan and B. Mathew, Review of β-carboline and its derivatives as selective MAO-A inhibitors, Arch. Pharm., 2023, 2300091, DOI:10.1002/ardp.202300091.
- S. Kumar, A. S. Nair, M. A. Abdelgawad and B. Mathew, Exploration of the Detailed Structure–Activity Relationships of Isatin and Their Isomers as Monoamine Oxidase Inhibitors, ACS Omega, 2022, 7(19), 16244–16259 CrossRef CAS.
- B. Mathew, J. M. Oh, M. A. Abdelgawad, A. Khames, M. M. Ghoneim, S. Kumar, L. R. Nath, S. T. Sudevan, D. G. T. Parambi, C. Agoni, M. E. S. Soliman and H. Kim, Conjugated Dienones from Differently Substituted Cinnamaldehyde as Highly Potent Monoamine Oxidase-B Inhibitors: Synthesis, Biochemistry, and Computational Chemistry, ACS Omega, 2022, 7(9), 8184–8197, DOI:10.1021/acsomega.2c00397.
- A. Bhawna, M. Kumar, A. Bhatia, P. Kapoor and S. Kumar, Monoamine oxidase inhibitors: A concise review with special emphasis on structure activity relationship studies, Eur. J. Med. Chem., 2022, 242, 114655, DOI:10.1016/j.ejmech.2022.114655.
- M. K. Burdina, E. Mateev, B. Angelov, V. Tzankova and M. Georgieva, In Silico Evaluation and In Vitro Determination of Neuroprotective and MAO-B Inhibitory Effects of Pyrrole-Based Hydrazones: A Therapeutic Approach to Parkinson's Disease, Molecules, 2022, 27(23), 8485, DOI:10.3390/molecules27238485.
- J. Suresh, S. C. Baek, S. P. Ramakrishnan, H. Kim and B. Mathew, Discovery of potent and reversible MAO-B inhibitors as furanochalcones, Int. J. Biol. Macromol., 2018, 108, 660–664, DOI:10.1016/j.ijbiomac.2017.11.159.
- D. G. T. Parambi, J. M. Oh, S. C. Baek, J. P. Lee, A. R. Tondo, O. Nicolotti, H. Kim and B. Mathew, Design, synthesis and biological evaluation of oxygenated chalcones as potent and selective MAO-B inhibitors, Bioorg. Chem., 2019, 93, 103335, DOI:10.1016/j.bioorg.2019.103335.
- R. Sasidharan, S. L. Manju, G. Uçar, I. Baysal and B. Mathew, Identification of Indole-Based Chalcones: Discovery of a Potent, Selective, and Reversible Class of MAO-B Inhibitors, Arch. Pharm., 2016, 349(8), 627–637, DOI:10.1002/ardp.201600088.
- B. Mathew, A. Haridas, G. Uçar, I. Baysal, A. A. Adeniyi, M. E. Soliman, M. Joy, G. E. Mathew, B. Lakshmanan and V. Jayaprakash, Exploration of chlorinated thienyl chalcones: A new class of monoamine oxidase-B inhibitors, Int. J. Biol. Macromol., 2016, 91, 680–695, DOI:10.1016/j.ijbiomac.2016.
- B. Mathew, A. Haridas, G. Uçar, I. Baysal, M. Joy, G. E. Mathew, B. Lakshmanan and V. Jayaprakash, Synthesis, Biochemistry, and Computational Studies of Brominated Thienyl Chalcones: A New Class of Reversible MAO-B Inhibitors, ChemMedChem, 2016, 11(11), 1161–1171, DOI:10.1002/cmdc.201600122.
- G. S. Jeong, M. G. Kang, J. Y. Lee, S. R. Lee, D. Park, M. Cho and H. Kim, Inhibition of butyrylcholinesterase and human monoamine oxidase-B by the coumarin glycyrol and liquiritigenin isolated from Glycyrrhiza uralensis, Molecules, 2020, 25(17), 3896, DOI:10.3390/molecules25173896.
- N. A. Rehuman, J. M. Oh, L. R. Nath, A. Khames, M. A. Abdelgawad, N. Ambacorta, O. Nicolotti, R. K. Jat, H. Kim and B. Mathew, Halogenated coumarin-chalcones as multifunctional monoamine oxidase-B and butyrylcholinesterase inhibitors, ACS Omega, 2021, 6(42), 28182–28193 CrossRef CAS PubMed.
- H. W. Lee, H. W. Ryu, M. G. Kang, D. Park, S. R. Oh and H. Kim, Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens, Bioorg. Med. Chem. Lett., 2016, 26(19), 4714–4719 CrossRef CAS PubMed.
-
Desmond, Schrödinger, https://www.schrodinger.com/platform/products/desmond/ (accessed 2024-06-07) Search PubMed.
- C. Binda, A. Mattevi and D. E. Edmondson, Structural properties of human monoamine oxidases A and B, Int. Rev. Neurobiol., 2011, 100, 1–11, DOI:10.1016/B978-0-12-386467-3.00001-7.
- S. Y. Son, J. Ma, Y. Kondou, M. Yoshimura, E. Yamashita and T. Tsukihara, Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(15), 5739–5744, DOI:10.1073/pnas.0710626105 , Epub 2008 Apr 7.
- S. Kumar, J. M. Oh, P. Prabhakaran, A. Awasti, H. Kim and B. Mathew, Isatin-tethered halogen-containing acylhydrazone derivatives as monoamine oxidase inhibitor with neuroprotective effect, Sci. Rep., 2024, 14(1), 1264, DOI:10.1038/s41598-024-51728-x , PMID: 38218887.
- A. Daina, O. Michielin and V. Zoete, SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci. Rep., 2017, 7, 42717, DOI:10.1038/srep42717.
- A. Daina and V. Zoete, A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules, ChemMedChem, 2016, 11(11), 1117–1121, DOI:10.1002/cmdc.201600182.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *b and
Bijo
Mathew
*b and
Bijo
Mathew
 *a
*a
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and a peak in the C13 NMR (range between 188–177 ppm) [ESI†].
O) and a peak in the C13 NMR (range between 188–177 ppm) [ESI†].








