Open Access Article
Open Access ArticleRAFT solution polymerisation of bio-based γ-methyl-α-methylene-γ-butyrolactone monomer in DMSO and Cyrene†
Oliver J.
Harris
 a,
Ryan R.
Larder
a,
Ryan R.
Larder
 a,
Beth
Jordan
a,
Imogen
Prior
a,
Rita
El-Khoury
a,
Khaled O.
Sebakhy
a,
Beth
Jordan
a,
Imogen
Prior
a,
Rita
El-Khoury
a,
Khaled O.
Sebakhy
 b and
Fiona L.
Hatton
b and
Fiona L.
Hatton
 *a
*a
aDepartment of Material, Loughborough University, Loughborough, LE11 3TU, UK. E-mail: f.hatton@lboro.ac.uk
bDepartment of Materials, Textiles and Chemical Engineering, Centre for Polymer and Material Technologies (CPMT), Ghent University, Ghent, Belgium
First published on 1st November 2024
Abstract
Reversible addition fragmentation chain transfer (RAFT) solution polymerisation of the bio-based lactone monomer γ-methyl-α-methylene-γ-butyrolactone (γMeMBL) has been demonstrated in DMSO and Cyrene. RAFT control was evidenced by control over molecular weights, low disperisites, and kinetic evaluation. Purified P(γMeMBL) homopolymers exhibited high glass transition temperatures (206–221 °C) and excellent thermal stabilities. This work demonstrates the first RAFT solution polymerisation of γMeMBL and the first example of RAFT polymerisation in Cyrene.
Concerns around sustainability of polymers have driven an increase in research into the preparation of new polymers derived from biomass.1 Recently, interest in a family of five-membered α-methylene-γ-butyrolactone monomers, bearing an exocyclic double bond, has grown as these monomers are analogous to methyl methacrylate (MMA) and can be derived from nature, see Fig. 1.2,3 For instance, α-methylene-γ-butyrolactone (MBL), also known as Tulipalin A, is found in Tulips. MBL and other analogues, β-methyl-α-methylene-γ-butyrolactone (βMeMBL)4 and α-methylene-γ,γ-dimethyl-γ-butyrolactone (Me2MBL)5 have been synthesised from itaconic acid, an important renewable feedstock converted from glucose.6 While γ-methyl-α-methylene-γ-butyrolactone (γMeMBL), also known as α-methylene-γ-valerolactone, has been prepared via a γ-valerolactone intermediate from levulinic acid, another biomass derived starting material.7 Others have also demonstrated the transformation of γ-valerolactone to γMeMBL in batch8 and flow reactors.9
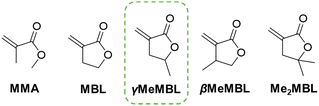 | ||
| Fig. 1 Chemical structures of MMA and the family of 5-membered α-methylene-γ-butyrolactone monomers; MBL, γMeMBL, βMeMBL and Me2MBL. | ||
The versatility of these monomers has been demonstrated through successful anionic, group transfer, free radical, reversible deactivation radical, and ring opening polymerisations.3,10–13 Ring opening polymerisation results in a polyester containing double bonds,14 while other polymerisation methods (anionic, radical, group transfer) proceed through the exocyclic double bond, retaining the five-membered ring structure within the polymer.
RDRP techniques such as reversible addition fragmentation chain transfer (RAFT) polymerisation, atom transfer radical polymerisation (ATRP) and nitroxide mediated polymerisation (NMP) have become popular techniques to synthesise well-defined polymers, including various architectures with control over molecular weights.15,16 The use of these techniques to synthesise new bio-based polymers using monomers derived from renewable resources has grown in recent years.17,18 However, RDRP investigations of this class of α-methylene-γ-butyrolactone monomers is limited to a few examples. MBL was polymerised by ATRP19–21 and NMP22 to generate well-defined homo- and block copolymers and has also been demonstrated in oxygen tolerant photochemically induced ATRP.23 RAFT polymerisations of these monomers have been investigated in the bulk, solution and miniemulsion polymerisations. Qi et al. reported RAFT bulk and miniemulsion polymerisations of γMeMBL in 2008, using cumyl dithiobenzoate as the chain transfer agent (CTA).24 While this CTA controlled the bulk polymerisations reasonably well, aggregation of latex was observed in miniemulsion. To overcome difficulties with the polymerisation of γMeMBL, styrene was employed as a comonomer. In 2013, Luo and coworkers reported the RAFT ab initio emulsion copolymerisation of γMeMBL and styrene, using a poly(acrylic acid)-b-(styrene) macromolecular chain transfer agent, reporting latex with up to 60 wt% γMeMBL.25 Trotta et al. have recently demonstrated the RAFT polymerisation of MBL and Me2MBL in benzene, using 2-cyano-2-propyl benzodithioate (CPDB) as the chain transfer agent.5 Recently, RAFT polymerisation of MBL using CPDB was reported in supercritical CO2 at 300 bar and 80 °C, reporting relatively high conversions (85%) after 24 h, compared with using DMF as the reaction solvent (∼65%).26 Thus, the RDRP of these monomers is limited and warrants further investigation.
While it is important to use renewable and bio-based materials, it is also imperative to focus on other reaction conditions to improve sustainability by applying Green Chemistry principles.27 For example, more sustainable solvents reported for RDRP polymerisations include 2-methyltetrahydrofuran,28,29 ethyl lactate,30–32 cyclopentyl methyl ether29,33 and ionic liquids.34 Cyrene (dihydrolevoclucosenone) is a solvent of interest as it has been reported as an alternative to polar solvents (DMSO, DMF, NMP), can be produced from biomass, is biodegradable, non-mutagenic and non-toxic.35 Recently, Cyrene has been reported as a suitable solvent for Cu catalysed RDRP polymerisations of (meth)acrylates36,37 and ring opening methathesis polymerisation of levoglucosenyl alkyl ethers.38 However, it is still unexplored as a solvent for RAFT polymerisation.
Herein, we report RAFT solution polymerisation of the bio-based lactone γMeMBL in DMSO and Cyrene. Initial RAFT polymerisation conditions were screened, including solvent, CTA, radical initiator, and CTA/In ratio. Once suitable reaction conditions were identified, P(MeMBL)x homopolymers were synthesised with varying degrees of polymerisation, kinetic experiments were conducted, and resulting polymers were fully characterised to reveal impressive thermal properties.
Initial screening was performed to identify suitable RAFT polymerisation conditions (Table S1, ESI†). In all experiments the targeted degree of polymerisation (DP) was 100, while the CTA, solvent and radical initiator were varied. In all cases the monomer conversion, as determined by 1H NMR (Fig. S1, ESI†), was relatively high (>85%). Average molecular weights (Mn, Mw) and molecular weight distributions (Đ) were determined by CHCl3 GPC analyses. Initially, reaction solvents investigated included DMSO, MeOH and t-butanol. DMSO proved to be an effective solvent and has been reported for the free radical polymerisation of γMeMBL previously.39 Three different CTAs were investigated, CPDB, 4-cyanopentanoic acid dithiobenzoate (CPADB), and 2-cyano-2-propyl dodecyl trithiocarbonate (CPDT). Under identical reaction conditions, CPDB gave a P(γMeMBL)100 homopolymer with the lowest dispersity, Đ, of 1.26, compared with 1.32 for CPABD and 1.41 for CPDT. Therefore, as CPDB appeared to facilitate the RAFT polymerisation of γMeMBL and gave P(γMeMBL) with a relatively low dispersity (Đ < 1.3) compared with CPADB and CPDT, it was used for all subsequent RAFT polymerisations. Previous reports of the RAFT polymerisation of MBL and Me2MBL also used CPDB, albeit in a more hazardous solvent (benzene).5 Using 4,4′-azobis(4-cyanovaleric acid) (ACVA) as the radical initiator resulted in slightly higher dispersity of 1.37 compared with 1.26 with azobisisobutyronitrile (AIBN), hence AIBN was used for the rest of the study. The selected polymerisation conditions in DMSO are detailed in Scheme 1.
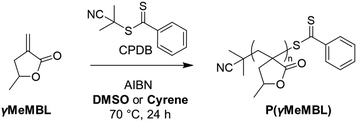 | ||
| Scheme 1 Reaction scheme for the RAFT solution polymerisation of γMeMBL with either DMSO or Cyrene as the solvent at 70 °C. | ||
To investigate the RAFT solution polymerisation of γMeMBL further, varying degrees of polymerisation (DP = 50–400) were targeted, see Table 1. The CPDB/AIBN ratio was maintained at 5, and the monomer concentration was 40% w/w. High conversions were obtained when targeting DPs between 50–200 (>92%), with a moderate reduction in conversion observed at the highest target DP of 400 (73%), likely caused by the reduced initiator concentration. The resulting P(γMeMBL)x homopolymers exhibited reasonably low dispersities (Đ = 1.26–1.31), see Fig. S2 (ESI†). Moreover, a linear increase in Mn by GPC was observed versus theoretical Mn (Fig. S3, ESI†).
| Target composition | Solvent | Conversiona (%) | M n th (g mol−1) | M n (g mol−1) | M w (g mol−1) | Đ |
|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy. b Theoretical Mn (Mn th) calculated as follows: Mn th = ((Mw mon × target DP) × (Conv/100)) + Mw CTA. c Determined by CHCl3 GPC analyses. | ||||||
| P(γMeMBL)50 | DMSO | 97 | 5700 | 5000 | 6600 | 1.31 |
| P(γMeMBL)100 | 94 | 10800 | 8300 | 10800 | 1.31 | |
| P(γMeMBL)200 | 92 | 20900 | 13500 | 16900 | 1.26 | |
| P(γMeMBL)400 | 73 | 33000 | 19800 | 25100 | 1.27 | |
| P(γMeMBL)50 | Cyrene | 95 | 5500 | 5700 | 7100 | 1.25 |
| P(γMeMBL)100 | 78 | 9000 | 7100 | 9400 | 1.32 | |
| P(γMeMBL)200 | 83 | 18800 | 12200 | 15100 | 1.24 | |
| P(γMeMBL)400 | 65 | 29400 | 18100 | 22100 | 1.22 | |
Following the RAFT polymerisation of γMeMBL in DMSO, we investigated the feasibility of performing these syntheses in the more sustainable solvent Cyrene. RAFT polymerisations were conducted in Cyrene using identical reaction conditions to those used in DMSO (see Scheme 1) targeting the same DP range of 50–400, see Table 1. Generally, slightly lower conversions were achieved compared with the equivalent polymerisations conducted in DMSO (65–95%). Control over the molecular weights was observed by GPC analyses (Fig. 2). As observed for the polymerisations in DMSO, the Mn by GPC for P(γMeMBL)x synthesised in Cyrene increased linearly with theoretical Mn (Fig. S3, ESI†) and dispersities, Đ, were relatively low (Đ ≤ 1.32).
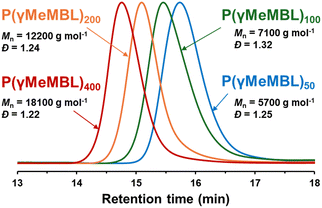 | ||
| Fig. 2 Normalised overlaid GPC chromatograms for P(γMeMBL)x synthesised by RAFT solution polymerisation of γMeMBL in Cyrene at 70 °C for 24 h, using CPDB. | ||
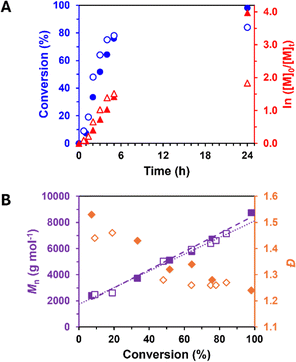
Kinetic evaluations were conducted during the syntheses of P(γMeMBL)100 in both DMSO and Cyrene, removing samples periodically to determine monomer conversion and molecular weight evolution during the RAFT solution polymerisation, see Fig. 3. First order kinetics were observed along with a linear increase in Mn with conversion, suggesting RAFT control of the polymerisation under these conditions.
It is worth noting that unreacted CPDB was observed by UV-GPC analyses for all samples (during the kinetic evaluations and varying DPs), which suggests that the CTA efficiency here was not optimal. Nevertheless, the presence of the RAFT end groups were confirmed for all P(γMeMBL)x homopolymers through GPC analyses, evidenced by the direct overlap of the UV absorption peak for the dithiobenzoate group (λmax = 290 nm) with the corresponding refractive index signal (Fig. S4, ESI†). Furthermore, the RAFT end group fidelity of P(γMeMBL)50 was investigated by chain extension with 50 more units of γMeMBL (Table S2, ESI†). The resulting P(γMeMBL)100 was found to have moderate dispersity (Đ = 1.45), increased Mn and a clear shift in the molecular weight distribution (Fig. S5, ESI†). Deconvolution of the raw RI detector response showed that approximately 20% unreacted P(γMeMBL)50 remained, suggesting a blocking efficiency of ~80%.
One important feature of α-methylene-γ-butyrolactone-based polymers is their impressive thermal properties, compared with PMMA, for example. The P(γMeMBL)x thermal properties were studied using differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA), see Table S3 (ESI†). DSC revealed the polymers exhibited glass transitions, Tg, of 206, 210, 218 and 221 °C for P(γMeMBL) with Mn of 5.7, 7.1, 12.2 and 18.1 kg mol−1 respectively, with a molecular weight dependence, see Fig. 4. The increase in Tg with increasing Mn is observed due to the reduction in free volume with increasing chain length, as per the Fox-Flory relationship.40 Similar Tg values (210–225 °C) were reported by Miyake et al., for P(γMeMBL) synthesised by coordination polymerisation, with Mn = 2.64–543 kg mol−1.41 This represents a marked increase over a typical high Tg methacrylate-based polymer poly(methyl methacrylate) (Tg = 105 °C).42 For all P(γMeMBL)n samples, a slight endothermic process is also observed towards the end of the Tg step transition, indicative of a enthalpy relaxation.43 TGA showed that the thermal decomposition of the polymers was nearly identical for all P(γMeMBL)x compositions, with an onset of degradation observed in the region of 345–366 °C, see Fig. S6 (ESI†). Again, this is appreciably higher than the comparable PMMA (typically in the range of 270–300 °C),44 and agrees with previous work where the onset of degradation of P(γMeMBL), synthesised by coordination polymerisation, was 356 °C.45
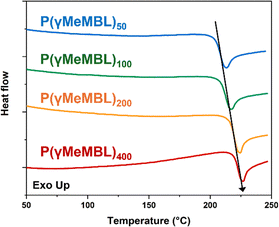 | ||
| Fig. 4 Offset DSC thermograms for P(γMeMBL)x synthesised by RAFT solution polymerisation of γMeMBL in Cyrene at 70 °C for 24 h, using CPDB. | ||
In summary, we report the successful RAFT solution polymerisation of the bio-based lactone monomer γMeMBL, and RAFT solution polymerisation using Cyrene, a more sustainable solvent. RAFT control was confirmed by kinetic evaluation, observation of RAFT chain end fidelity and control over target molecular weights and dispersities. However, CTA selection could be improved in future work. The resulting P(γMeMBL)x homopolymers had high glass transition temperatures (>200 °C), with molecular weight dependence observed, and excellent thermal stabilities compared with PMMA. This work demonstrates a move towards both well-defined renewable polymers and more sustainable RAFT polymerisation protocols which are both essential for producing sustainable well-defined polymers for value-added applications.
This work was funded by the EPSRC (FLH, EP/W019175/1; OH and RRL, EP/T518098/1) and Loughborough University. We also thank the Department of Chemistry at Loughborough University for access to NMR Spectroscopy.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef PubMed.
- R. R. A. Kitson, A. Millemaggi and R. J. K. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426–9451 CrossRef PubMed.
- J. Kollár, M. Danko, F. Pippig and J. Mosnáček, Front. Chem., 2019, 7, 1–8 CrossRef PubMed.
- R. R. Gowda and E. Y. X. Chen, Org. Chem. Front., 2014, 1, 230–234 RSC.
- J. T. Trotta, M. Jin, K. J. Stawiasz, Q. Michaudel, W.-L. Chen and B. P. Fors, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2730–2737 CrossRef.
- J. Becker, A. Lange, J. Fabarius and C. Wittmann, Curr. Opin. Biotechnol, 2015, 36, 168–175 CrossRef.
- L. E. Manzer, Appl. Catal., A, 2004, 272, 249–256 CrossRef.
- Z. Vobecka, C. Wei, K. Tauer and D. Esposito, Polymer, 2015, 74, 262–271 CrossRef.
- M. Al-Naji, B. Puértolas, B. Kumru, D. Cruz, M. Bäumel, B. V. K. J. Schmidt, N. V. Tarakina and J. Pérez-Ramírez, ChemSusChem, 2019, 12, 2628–2636 CrossRef PubMed.
- V. Graur, A. Mukherjee, K. O. Sebakhy and R. K. Bose, Polymers, 2022, 14, 3993 CrossRef CAS.
- A. Elshewy, M. El Hariri El Nokab, J. E. Sayed, Y. A. Alassmy, M. M. Abduljawad, D. R. D’hooge, P. H. M. Van Steenberge, M. H. Habib and K. O. Sebakhy, ACS Appl. Polym. Mater., 2024, 6, 115–125 CrossRef CAS.
- M. K. Akkapeddi, Macromolecules, 1979, 12, 546–551 CrossRef CAS.
- M. Mousa, H. Bergenudd, A. L. Kron and E. Malmström, Macromolecules, 2021, 54, 6127–6134 CrossRef CAS.
- M. Danko, M. Basko, S. Ďurkáčová, A. Duda and J. Mosnáček, Macromolecules, 2018, 51, 3582–3596 CrossRef CAS.
- M. Destarac, Polym. Chem., 2018, 9, 4947–4967 RSC.
- N. Corrigan, K. Jung, G. Moad, C. J. Hawker, K. Matyjaszewski and C. Boyer, Prog. Polym. Sci., 2020, 111, 101311 CrossRef CAS.
- F. L. Hatton, Polym. Chem., 2020, 11, 220–229 RSC.
- M. Palà, S. E. Woods, F. L. Hatton and G. Lligadas, Macromol. Chem. Phys., 2022, 2200005 CrossRef.
- J. Mosnáček and K. Matyjaszewski, Macromolecules, 2008, 41, 5509–5511 CrossRef.
- J. Shin, Y. Lee, W. B. Tolman and M. A. Hillmyer, Biomacromolecules, 2012, 13, 3833–3840 CrossRef PubMed.
- P. Verdugo, G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, Eur. Polym. J., 2021, 147, 110321 CrossRef.
- A. Métafiot and M. Marić, J. Polym. Sci., 2024, 62, 3399–3413 CrossRef.
- G. Zain, D. Bondarev, J. Doháňošová and J. Mosnáček, ChemPhotoChem, 2019, 3, 1138–1145 CrossRef.
- G. Qi, M. Nolan, F. J. Schork and C. W. Jones, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5929–5944 CrossRef.
- S. Xu, J. Huang, S. Xu and Y. Luo, Polymer, 2013, 54, 1779–1785 CrossRef.
- F. G. Versteeg, N. C. Hegeman, K. O. Sebakhy and F. Picchioni, Macromol. Rapid Commun., 2022, 43, 1–7 CrossRef PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2009, 39, 301–312 RSC.
- G. Englezou, K. Kortsen, A. A. C. Pacheco, R. Cavanagh, J. C. Lentz, E. Krumins, C. Sanders-Velez, S. M. Howdle, A. J. Nedoma and V. Taresco, J. Polym. Sci., 2020, 58, 1571–1581 CrossRef.
- K. Parkatzidis, S. Boner, H. S. Wang and A. Anastasaki, ACS Macro Lett., 2022, 11, 841–846 CrossRef CAS.
- A. Moreno, D. Garcia, M. Galià, J. C. Ronda, V. Cádiz, G. Lligadas and V. Percec, Biomacromolecules, 2017, 18, 3447–3456 CrossRef CAS PubMed.
- A. Moreno, N. Bensabeh, J. Parve, J. C. Ronda, V. Cádiz, M. Galià, L. Vares, G. Lligadas and V. Percec, Biomacromolecules, 2019, 20, 1816–1827 CrossRef PubMed.
- N. Bensabeh, A. Moreno, A. Roig, O. R. Monaghan, J. C. Ronda, V. Cádiz, M. Galià, S. M. Howdle, G. Lligadas and V. Percec, Biomacromolecules, 2019, 20, 2135–2147 CrossRef PubMed.
- C. M. R. Abreu, P. Maximiano, T. Guliashvili, J. Nicolas, A. C. Serra and J. F. J. Coelho, RSC Adv., 2016, 6, 7495–7503 RSC.
- G. Durga, P. Kalra, V. Kumar Verma, K. Wangdi and A. Mishra, J. Mol. Liq., 2021, 335, 116540 CrossRef.
- N. A. Stini, P. L. Gkizis and C. G. Kokotos, Green Chem., 2022, 24, 6435–6449 RSC.
- A. Marathianos, E. Liarou, E. Hancox, J. L. Grace, D. W. Lester and D. M. Haddleton, Green Chem., 2020, 22, 5833–5837 RSC.
- I. Zaborniak, M. Klamut, C. M. Warne, K. Kisiel, M. Niemiec, P. Błoniarz, A. Pellis, K. Matyjaszewski and P. Chmielarz, ACS Sustainable Chem. Eng., 2024, 12, 4933–4945 CrossRef PubMed.
- T. Debsharma, B. Schmidt, A. Laschewsky and H. Schlaad, Macromolecules, 2021, 54, 2720–2728 CrossRef.
- M. Van Den Brink, A. M. Van Herk and A. L. German, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 3793–3803 CrossRef.
- T. G. Fox and P. J. Flory, J. Appl. Phys., 1950, 21, 581–591 CrossRef.
- G. M. Miyake, Y. Zhang and E. Y.-X. Chen, Macromolecules, 2010, 43, 4902–4908 CrossRef.
- E. A. Grulke, E. H. Immergut and J. Brandrup, Polymer handbook, Wiley, 2004 Search PubMed.
- M. J. Parker, in Comprehensive Composite Materials, Elsevier, 2000, pp. 183–226 Search PubMed.
- M. Ferriol, A. Gentilhomme, M. Cochez, N. Oget and J. L. Mieloszynski, Polym. Degrad. Stab., 2003, 79, 271–281 CrossRef.
- G. M. Miyake, S. E. Newton, W. R. Mariott and E. Y.-X. Chen, Dalton Trans., 2010, 39, 6576–6588 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, RAFT polymerisation screening data, 1H NMR analyses of P(γMeMBL)x and γMeMBL monomer, GPC chromatograms for P(γMeMBL)x synthesised in DMSO, Mn by GPC vs. Theoretical Mn plot, overlaid RI and UV detector GPC chromatograms, conversions, molecular weight data and GPC chromatograms from chain extension experiment, glass transition temperatures, onset of thermal degradation and TGA profiles for purified P(γMeMBL)x synthesised in Cyrene. See DOI: https://doi.org/10.1039/d4cc04571h |
| This journal is © The Royal Society of Chemistry 2024 |