Open Access Article
Open Access ArticleNanozymes: a promising solution for dental antibacterial applications
Lipeng Liu†
 ab,
Yaoyuan Zhang†a,
Tianjuan Jua,
Xutao Chenb,
Xinwei Lia and
Li-an Wu*a
ab,
Yaoyuan Zhang†a,
Tianjuan Jua,
Xutao Chenb,
Xinwei Lia and
Li-an Wu*a
aState Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, National Clinical Research Center for Oral Diseases, Shaanxi Clinical Research Center for Oral Diseases, Department of Pediatric Dentistry, School of Stomatology, The Fourth Military Medical University, China. E-mail: lianwu@fmmu.edu.cn
bDepartment of Immunology, School of Basic Medicine, The Fourth Military Medical University, China
First published on 20th November 2024
Abstract
Dental diseases pose significant public health challenges globally, affecting millions with conditions exacerbated by microbial-induced inflammation. Traditional natural enzymes, despite their antibacterial and anti-inflammatory capabilities, are limited by operational stability and environmental sensitivity. This review explores the revolutionary realm of nanozyme-artificial enzymes made from nanomaterials-which offer enhanced stability, cost-effectiveness, and ease of modification. We discuss the advent of nanozymes since their first recognition in 2007, emphasizing their enzyme-mimicking capabilities and applications in dental medicine, particularly for dental caries, pulpitis, periodontitis and peri-implantitis. This paper presents a comprehensive analysis of nanozymes' classification, mechanisms, and emerging applications, shedding light on their potential to revolutionize dental antibacterial treatments and addressing current challenges and future perspectives in their development.
1. Introduction
Dental diseases are among the most common diseases globally, posing significant health and economic challenges and substantially diminishing the quality of life for those impacted.1 Microbial-induced inflammation is a common feature of dental diseases like dental caries, endodontic diseases, periodontitis, and peri-implantitis.2 Mechanical decontamination is a commonly used clinical method that effectively removes plaque biofilms. Nevertheless, it has limitations in cleaning inaccessible areas and can damage tooth and implant surfaces, promoting bacterial aggregation and resulting in bleeding and injury to the alveolar bone and gums.3 Nonmechanical approaches, such as antiseptics like chlorhexidine and hydrogen peroxide, can effectively penetrate the microstructures of implant fixtures without damaging surfaces. However, chlorhexidine is less effective against plaque biofilms and is unsuitable for daily use due to adverse effects like tartar formation and tooth staining, while high-concentration hydrogen peroxide can harm normal tissues.4,5 The supplemental use of local antibiotics offers an additional treatment option for oral infectious diseases. Yet, eliminating bacterial biofilms in vivo at minimal antibiotic concentrations is challenging. Additionally, high doses of antibiotics can increase biofilm tolerance and promote bacterial drug resistance.6 Promising research and applications of natural enzymes with antibacterial properties have been explored for treating these conditions.7 However, natural enzymes often suffer from intrinsic limitations, including low operational stability, sensitivity to temperature and pH variations, and difficulties in recycling.8 To overcome these deficiencies, researchers have explored enzyme mimics.Nanozymes, as artificial enzymes, are a new type of functional nanomaterial with enzyme mimic activities.9 Compared to natural enzymes, nanozymes are easier to prepare, have adjustable catalytic activity, exhibit high stability, are cost-effective, and are more manageable.10 These attributes position nanozymes as viable substitutes for natural enzymes across industrial, biological, and medical fields.11 Since the discovery of horseradish peroxidase(POD)-like activity in ferromagnetic nanoparticles in 2007,12 numerous nanozymes have been synthesized and utilized in various fields.13,14 In 2013, Wei and colleagues described nanozymes as nanomaterials exhibiting enzyme-like activities.15 Currently, nanozymes are acknowledged for their ability to catalyze substrates into products under conditions similar to those of traditional artificial enzymes.16 The unique magnetic, fluorescent, and electrical properties of nanozymes further enhance their potential as substitutes for natural enzymes, making them a focal point of recent research and development.15
Over the past decade, nanozymes have driven significant technological advancements in oral medicine due to their exceptional physicochemical properties and intrinsic enzyme-like activities.17 They offer promising solutions for treating conditions such as caries, periodontitis, and peri-implantitis. Despite advancements, comprehensive reviews on nanozyme applications in dental antibacterial research are limited. Previous reviews have made substantial contributions but still exhibit some limitations. For instance, Chen et al. conducted an extensive review on the application of nanozymes in oral healthcare, discussing various aspects including antibacterial, anti-inflammatory, and tissue regeneration properties.17 Notably, their classification approach concentrated on diseases related to antibacterial applications; however, their work did not specifically address the use of nanozymes in periodontal disease treatment. Cai et al. focused their review on the use of nanozymes for treating oral infections, highlighting their antibacterial and anti-inflammatory properties.18 However, their review lacked a comprehensive overview of antibacterial applications, as it covered only a limited number of cases. This selective approach may be less informative for newcomers to the field who are looking for a more complete understanding. As nanozymes were recognized as one of the top ten chemical advancements of 2022,19 there has been rapid development and an influx of new research, especially in the field of dental antibacterial applications. It is worth noting that the reviews by Chen and Cai were both published before 2023, and no comprehensive review has yet covered the significant advancements made in this field over the past two years. To address these gaps and provide an up-to-date perspective, a thorough overview and analysis of recent research on nanozymes for dental antibacterial applications is necessary. Our work makes several key contributions compared to existing literature: first, we provide an extensive analysis of the use of nanozymes in dental antibacterial treatment, specifically focusing on applications in dental caries, pulpitis, periodontitis, and peri-implantitis, thereby offering a holistic view of their potential in managing diverse dental conditions. Furthermore, we incorporate the most recent advancements, including publications from the past two years, such as the emerging use of single-atom nanozymes for implant-related biofilm infections—an area that previous reviews have not adequately covered. In contrast to earlier reviews that often discussed antibacterial, anti-inflammatory, and tissue regeneration aspects together, our work focuses exclusively on the direct antibacterial action of nanozymes. This focused approach allows for a detailed and in-depth exploration of their antibacterial efficacy, without delving into broader combined applications or anti-inflammatory contexts.
This review elucidates the classification, catalytic mechanisms, and significant progress of nanozymes in dental medicine. It begins by categorizing nanozymes based on metal elements and enzymatic reaction mechanisms, and then explains their antibacterial mechanisms. Importantly, the review details the applications of nanozymes in dental antibacterial fields (Table 1), providing an in-depth overview of their potential in treating dental caries, pulpitis, periodontitis, and peri-implantitis. The paper concludes by outlining the challenges associated with nanozymes in dental medicine and suggesting directions for future research. This review aims to inspire new insights and technologies, providing readers with a comprehensive understanding and outlook for developing more effective antibacterial nanozymes, thus advancing dental antibacterial research.
| Application | Nanozyme formulations | Enzyme-like activity | Functionality | Ref. |
|---|---|---|---|---|
| Caries | Fe3O4 | POD | Degrade exopolysaccharides and kill bacteria disrupt intractable oral biofilms and prevent tooth decay | 20 |
| Ferumoxytol | POD | 21 | ||
| Ferumoxytol | POD | Disrupt cell membrane and degrade EPS matrix | 22 | |
| Ferumoxytol/SnF2 | POD | Inhibit biofilm accumulation and decrease enamel damage | 23 | |
| Graphdiyne/L-cys/Ag | POD and CAT | Remove plaque biofilm and remineralize teeth | 24 | |
| Fe3O4/dextran | POD | Target biofilm cells and degrade EPS matrix | 25 | |
| Fe3O4/dextran/GOx | GOx and POD | Increase H2O2, kill bacteria and degrade the EPS matrix | 26 | |
| CoPt@G@GOx | GOx and POD | Causes the death of bacteria both in planktonic states and within biofilms | 27 | |
| Iron oxide and iron sulfide | POD | Produce H2O2, degrade biofilm matrix and kill bacteria | 28 | |
| CaO2/TA/Fe | POD | Blast the tight biofilm and proceed with cascade catalysis eradication of biofilm | 29 | |
| Endodontic infections | Fe3O4 | POD | Enhance antibacterial activity on root canal surfaces and in dentinal tubules | 30 |
| Fe3O4/CaO2 | POD | Scavenge on root canal biofilm infection and prevent further inflammation expansion | 31 | |
| Fe3O4/GOx | GOx and POD | Eliminate E. faecalis and C. albicans and destructed the dense biofilm matrix | 32 | |
| Cu2+ | POD | Eradicate biofilms caused by E. faecalis and C. albicans in the root canals of infected teeth | 33 | |
| MPN-Pd | OXD | Inhibit biofilms formed by bacteria, fungi, and polymicrobial communities | 34 | |
| Iron oxide | POD | Kill bacteria and degrade and remove biofilms | 35 | |
| Periodontal disease | Oxygenated nanodiamonds | POD | Destruct bacterial cell membranes and biofilms | 36 |
| CN-PtNCs | OXD and POD | Alleviate inflammation and mitigate bone loss | 37 | |
| FeSN | POD | Decrease GSH and ATP and enhance bacterial killing efficiency | 38 | |
| Au/Pt NCs@GOX | GOx and POD | Disrupt biofilms and kill bacteria | 39 | |
| Fe3O4@Ce6/C6@MnO2 | CAT | Provide oxygen in infection sites and inhibit anaerobic pathogens | 40 | |
| CaO2/MnO2 | CAT | Provide a continuous oxygen supply and enhance the potential for periodontal healing | 41 | |
| CeO2@Ce6 NPs | SOD and CAT | Eradicate bacteria and mitigate inflammation | 42 | |
| Lu-Bi2Te3@Fe3O4 | POD | Cause nitrosative stress on biomacromolecules and damage bacterial cell membranes and DNA | 43 | |
| Peri-implantitis | MnO2 | CAT | Entirely destroy biofilms without harming the surrounding mucosa or implant surfaces | 44 |
| Ce-MOF | OXD | Consume extracellular ATP, inhibit bacterial adhesion and prevent biofilm formation | 45 | |
| CuNx-CNS SAzyme | POD, OXD and CAT | Inhibit multidrug-resistant bacteria and eliminate stubborn biofilms | 46 | |
| Cu2MoS4 | POD, OXD and CAT | Kill bacteria, polarize macrophages and promotes healing of infected tissue | 47 |
2. Classification of nanozymes
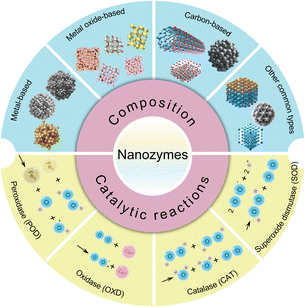
Nanozymes, a novel type of nanomaterial, exhibit inherent enzyme-like properties, making them particularly valuable in antibacterial applications. These materials are renowned for their catalytic activities, stability, and versatility. Depending on their composition, nanozymes can be categorized into four primary types: metal-based, metal oxide-based, carbon-based, and other varieties (Fig. 1). Metal-based nanozymes include materials such as Au,39 Ag,48 and Pt,49 as well as their composites. They are known for their POD-like activities, which help disrupt bacterial biofilms and inhibit the growth of oral pathogens. Metal oxide-based nanozymes, including Fe3O4,50 CeO2,45 MnO2,41 and Co3O4,51 are composed of transition metal oxides and utilize Fenton reactions and charge transfer mechanisms to demonstrate enzyme-like activities. Carbon-based nanozymes, such as carbon dots, carbon nanotubes, graphdiyne, and MXenes, are favoured over metal-based nanozymes for their enhanced biocompatibility.52 However, there are currently no reports of their application in the field of oral antibacterial use. Other common types of nanozymes include metal–organic frameworks,45 metal sulfides,38 and Prussian blue53 nanozymes, which also mimic natural enzyme functions.Nanozymes are also classified based on the types of catalytic reactions they facilitate (Fig. 1). Nanozymes with POD activity, such as those based on Fe3O4, catalyze the breakdown of hydrogen peroxide (H2O2) into highly reactive hydroxyl radicals (˙OH).52 Oxidase (OXD) nanozymes, such as those based on Co, Ru, Au, and CeO2, efficiently catalyze substrate oxidation without requiring H2O2, even at low substrate concentrations.54 Catalase (CAT) nanozymes break down H2O2 into water and oxygen, reducing reactive oxygen species (ROS) accumulation and protecting cells from oxidative stress.55 Superoxide dismutase (SOD) nanozymes, including those based on CeO2, MnO2, Pt, and Prussian blue, effectively scavenge excess ROS, contributing to antioxidant defense. This activity can synergize with antibacterial effects, offering anti-inflammatory benefits for oral health.56 Additionally, nanozymes with hydrolase activity, which degrade extracellular DNA (eDNA), also contribute to antibacterial effects.57
3. Antibacterial mechanisms of nanozymes
The study of nanozymes has attracted significant attention from researchers. Understanding nanozyme-mediated antimicrobial mechanisms is crucial for improving infectious disease control and developing biomedical technologies. The antibacterial mechanisms of nanozymes remain underexplored due to the diversity of nanozyme types, their physical and chemical properties, and various interfering factors. Current findings categorize the antibacterial effects of nanozymes into two primary types: the generation of ROS and non-ROS mechanisms.Nanozymes primarily exhibit antibacterial effects by mimicking the POD and OXD activities of natural enzymes, leading to the generation and regulation of ROS. ROS are intermediate chemical species formed during the partial reduction of oxygen, encompassing H2O2, ˙OH, superoxide anions (˙O2−), and singlet oxygen (1O2).58 ROS can irreversibly damage bacterial structures, including cell walls, membranes, DNA, proteins, polysaccharides, and nucleic acids.59 Additionally, they can disintegrate mature biofilms and inhibit their formation. H2O2 has intrinsic antibacterial properties at high concentrations (166 mM to 1.0 M) but can also damage healthy tissues.54 However, nanozymes with POD-like activity can transform low concentrations of H2O2 (<1 mM) into highly toxic ˙OH, effectively eliminating bacteria.60 To further reduce reliance on H2O2, nanozymes with OXD-like activity have been developed. These nanozymes catalyze oxygen into H2O2 and highly reactive ˙O2−/1O2, demonstrating potent antibacterial capabilities.37
In addition to generating ROS, nanozymes can also generate reactive nitrogen species (RNS), which harm cells and demonstrate potent antibacterial effects against various bacteria, including resistant strains.43 Furthermore, nanozymes can mimic DNase-like activity, accelerating the hydrolysis of DNA and eliminating biofilms.61 eDNA, a key component of the extracellular matrix (ECM), helps bacteria adhere to surfaces and connect with each other, maintaining biofilm integrity.62 DNase nanozymes disrupt the ECM, significantly enhancing traditional antibiotics' efficacy against enclosed bacteria and offering a promising strategy to combat drug-resistant bacteria. In addition to enzyme-activity mechanisms, nanozymes catalyze oxygen production from H2O2, creating an aerobic environment that enhances antibacterial effects against anaerobic bacteria.40,63 Moreover, the mechanical force from the rapid movement of oxygen produced through the catalysis of H2O2 can disrupt and decontaminate biofilms, thereby exerting an antibacterial effect.44
4. Application of nanozymes in dental antibacterial field
According to research, there are approximately 700 types of microorganisms in the human mouth.64,65 Various microorganisms in the oral cavity form a closely related ecosystem with the host, and the microbial community is interconnected with the extracellular matrix to form a biofilm on the surface of the oral cavity.66,67 When the oral environment changes, the imbalance of the microbial ecosystem, in which the level of bacterial flora changes, may result in various oral diseases, including caries and periodontal disease, etc.68,69 However, conventional antibacterial agents have poor removal effect on dental plaque biofilms, and studies have found that the proportion of oral antimicrobial resistant bacteria gradually increases.70 Hence, there is a pressing need for novel clinical treatments to combat biofilms. Nanozyme-based antibacterial agents have gained significant attention in dentistry due to their cost-effectiveness, structural stability, exceptional antibacterial performance, and broad antibacterial spectrum.71 In this section, we explore how nanozymes can be utilized as a tool for the treatment and prevention of dental infections (Fig. 2).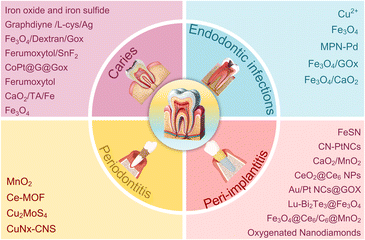 | ||
| Fig. 2 Application of nanozymes in dental antibacterial field (caries, endodontic infections, periodontitis and peri-implantitis). | ||
4.1 Caries
Dental caries is a prevalent bacterial infection, affecting up to 90% of school children and nearly all adults worldwide.72 Dental caries primarily results from the colonization and biofilm formation of pathogenic microorganisms on tooth surfaces.73 The protective extracellular matrix embedding bacteria makes dental biofilms difficult to remove or treat. Biofilms create acidic microenvironments that dissolve enamel apatite, resulting in dental caries.74 Traditional antimicrobials, such as chlorhexidine, often fail due to their limited effectiveness against cariogenic biofilms.75,76 Therefore, more potent antibiofilm treatments are necessary for caries prevention.In 2016, Gao et al. first reported a novel strategy using Fe3O4 nanozymes to manage plaque biofilms and prevent dental caries (Fig. 3A). The study demonstrated that Fe3O4 nanoparticles possess POD-like activity, converting H2O2 into free radicals in acidic conditions, which degrades exopolysaccharides and kills bacteria. This process mitigates dental caries severity and can halt its progression while preserving normal tissue in vivo.20 Both Fe3O4 and ferumoxytol are iron oxide nanoparticles (IONPs) with comparable catalytic activities crucial for their therapeutic effects. Ferumoxytol, approved by the US Food and Drug Administration for treating iron deficiency, has also been shown to inhibit tumor growth in mice by enhancing macrophage-associated ROS production.21,77 Inspired by the above research, Liu et al. discovered that ferumoxytol binds within the biofilm ultrastructure and generates free radicals from H2O2, which disrupt cell membranes and degrade the extracellular polymeric substances matrix, resulting in in situ bacterial death.21 Subsequent research presents initial human evidence supporting the therapeutic potential of catalytic IONPs as targeted nanomedicine for oral infectious diseases. It also demonstrates ferumoxytol's antimicrobial specificity against Streptococcus mutans, attributed to the interaction between ferumoxytol's carboxymethyl glucan and the glucan-binding protein of Streptococcus mutans.22 Ferumoxytol effectively disrupts caries-causing biofilms by catalytically activating H2O2, without affecting enamel acid demineralization. To improve the efficacy of ferumoxytol, Huang et al. discovered significant synergy when ferumoxytol is combined with stannous fluoride (SnF2), greatly inhibiting biofilm accumulation and enamel damage more effectively than either agent alone (Fig. 3B). Additionally, the study demonstrates that SnF2 enhances ferumoxytol's catalytic activity, significantly boosting ROS generation and antibiofilm efficacy.23 On the other hand, Liao et al. explored a different avenue for mineralization by developing a bioinspired ointment (Fig. 3C). They engineered graphdiyne/L-cysteine/Ag (GLA)nanozymes by anchoring low-dose Ag nanoparticles and ions on graphdiyne via a coordination-reduction strategy using L-cysteine. Encapsulated in a gelatin methacryloyl (GelMA) and sodium alginate (SA) base, the GLA/GS ointment activates under acidic conditions typical of dental plaque, converting low-dose H2O2 into highly reactive ˙OH. Furthermore, it facilitates enamel remineralization by providing nucleation sites that attract calcium and phosphate ions from saliva, promoting the growth of hydroxyapatite, the main component of tooth enamel.24
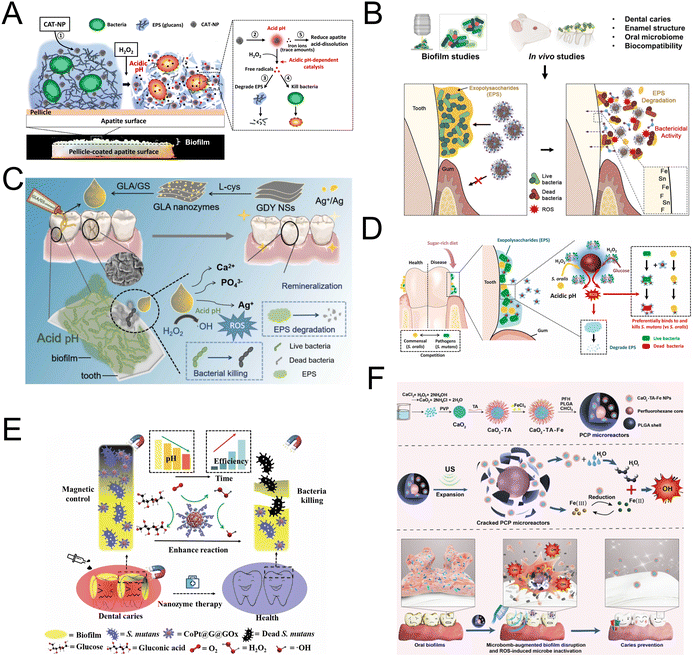 | ||
| Fig. 3 (A) Schematic diagram of biofilm disruption under acidic conditions by CAT-NP/H2O2 in situ. Reproduced with permission from ref. 20. Copyright 2016, Elsevier. (B) Schematic diagram of enhanced bioactivity and caries-protective effects against biofilms using laboratory and in vivo models. Reproduced with permission from ref. 23. Copyright 2023, Springer Nature. (C) Schematic diagram of synthesis of GLA/GS ointment using coordination-reduction combined biomineralization strategy and the catalytic ion therapy function for caries prevention. Reproduced with permission from ref. 24. Copyright 2024, Elsevier. (D) Schematic diagram of the selective catalytic−therapeutic mechanism of Dex-IONP-GOx for treatment of virulent acidogenic biofilms. Reproduced with permission from ref. 26. Copyright 2024, Elsevier. (E) Proposed mechanism of a hybrid nanozyme targeting oral pathogenic biofilms with antibacterial and antibiofilm effects through enzyme-nanozyme cascade reaction. Reproduced with permission from ref. 27. Copyright 2022, Springer Nature. (F) Proposed concept of the ultrasound-activated ROS generating microbombs targeting dental biofilm. Reproduced with permission from ref. 29. Copyright 2023, John Wiley and Sons. | ||
Considering that nanozymes lacked the stabilizing coating necessary for clinical applications, Naha et al. constructed bifunctional dextran-coated iron oxide nanozymes (Dex-NZM). Dex-NZM targets Streptococcus mutans in oral biofilms with high specificity through a dextran binding mechanism, achieving a selective antibacterial effect to prevent dental caries without affecting surrounding tissues.25,78 Pathogens like Streptococcus mutans flourish in sugar-rich environments and promote cariogenic biofilms.79 Based on Naha's earlier study, Huang et al. developed a bi-functional nanohybrid system with glucose oxidase (GOx) covalently attached to dextran-coated iron oxide nanoparticles (Fig. 3D). This system exploits disease-related pathological conditions (e.g., high sugar availability, low pH, and elevated EPS levels) to enhance H2O2 production and more effectively trigger ROS in a controlled, pH-dependent manner.26 Building on the advancements made by Naha et al. and Huang et al. in utilizing dextran-coated iron oxide nanoparticles for targeted antibiofilm action, Dong et al. expanded the application of nanozymes into a more integrated system (Fig. 3E). They developed CoPt@graphene@GOx (CoPt@G@GOx), a complex that combines GOx with magnetic graphitic CoPt nanocrystals (CoPt@G). In this configuration, GOx converts glucose present in the environment into gluconic acid and H2O2. The CoPt@G component, acting as a POD mimic, then utilizes the H2O2 generated by GOx to create highly toxic ˙OH. This mechanism effectively causes the death of bacteria both in planktonic states and within biofilms, leveraging the ROS in a manner similar to the earlier studies but with a unique, integrated approach that enhances efficacy and specificity.27
To address the issues of rapid H2O2 depletion and poor stability, which restrict sustained therapeutic effects in dental caries infected with biofilms, Wang et al. introduced an alternative strategy. Their research utilized Streptococcus gordonii themselves to produce H2O2. Utilizing iron oxide and iron sulfide nanozymes with POD-like activity, the researchers leveraged bacterial metabolism to produce H2O2, thereby introducing a novel approach to stabilize H2O2 and reduce Streptococcus mutans biofilm formation on human dentin surfaces.28 Many studies on nanozymes in antimicrobial biofilms have demonstrated significant reductions in biofilm formation and damage to biofilm structures. However, most of these studies do not employ clinically convenient treatment protocols or address the specificities of biofilms in particular environments, restricting their clinical applicability and everyday use for oral biofilm elimination. To address this gap, Guo et al. developed an ultrasonically-activated microbomb for dental biofilm elimination, incorporating tannic acid-iron modified calcium peroxide (CaO2-TA-Fe) nanoparticles and perfluorohexane (PFH) into poly(lactide-co-glycolide) (PLGA) (Fig. 3F). The ultrasonic toothbrush activates the PFH, inducing a phase shift that compromises the PLGA shell and triggers the release of H2O2 from CaO2. The tannic acid-iron network converts H2O2 into highly toxic ˙OH via the Fenton reaction, enhancing antibacterial effectiveness. This strategy is promising for cost-effective and widespread prevention of caries and treatment of biofilm-associated diseases.29
4.2 Endodontic infections
Endodontic infections are a common issue in dental medicine, frequently causing discomfort and clinical conditions like pain, swelling, pulpitis, apical periodontitis, and root resorption.80 The disinfection process is challenging due to the complex root canal system, which includes isthmuses, accessory canals, and dentinal tubules that can harbor bacteria and biofilms.81 Traditional disinfectants, including Ca(OH)2, sodium hypochlorite, and chlorhexidine, often fall short in effectively eliminating biofilms and are known to have certain adverse effects.82 Recent advancements in nanotechnology have opened up promising avenues for effectively eliminating bacteria, disrupting biofilms, and managing infections within dentinal tubules.83Biocompatible iron oxide nanoparticles exhibit potent antibiofilm properties without adverse effects on oral tissues in vivo.20 Bukhari et al. utilized iron oxide nanoparticles, with intrinsic POD-like activity to catalyze H2O2 and generate ROS, offering a novel endodontic disinfection method to enhance bacterial elimination in dentinal tubules.30 The effectiveness of Fe3O4 nanoparticles in biofilm control is often limited by low concentrations of H2O2 in microenvironments, and adding exogenous H2O2 could disrupt tissue healing. Addressing this, Song et al. developed a Fe3O4–CaO2 hydrogel that produces ROS in response to the bacterial environment, effectively eradicating root canal biofilm without requiring additional excitation (Fig. 4A).31 To enhance the production of H2O2, Ji et al. utilized GOx, an enzyme that catalyzes the conversion of β-D-glucose into H2O2, using molecular oxygen as the electron acceptor (Fig. 4B). This innovative method not only enables localized production of H2O2 but also depletes crucial energy sources for bacterial survival. It has shown significant antibacterial effectiveness against the Gram-positive bacterium Enterococcus faecalis and the yeast Candida albicans, highlighting its potential as a targeted antimicrobial strategy.32 Ethylenediaminetetraacetic acid (EDTA) is widely used as an irrigation solution, but its antimicrobial properties are limited.84 Aslan et al. engineered EDTA nanoformulations that exhibit catalytic and antimicrobial activities through a Fenton-like reaction with H2O2. EDTA nanofibers were used as an irrigation solution to eliminate biofilms formed by Enterococcus faecalis and Candida albicans in infected root canals.33 Photothermal therapy (PTT) can disrupt pathogen integrity by inducing localized hyperthermia through noninvasive light irradiation. Chen et al. developed metal–phenolic networks with palladium nanoparticle nodes, integrating OXD-like and photothermal properties to effectively inhibit biofilms formed by bacteria, fungi, and polymicrobial communities (Fig. 4C).34 By establishing root canal and oropharyngeal candidiasis models, they demonstrated the significant efficacy of this system in combating infections associated with such biofilms. In addressing the limitations inherent in both chemical and biological strategies, Hwang et al. introduced catalytic antimicrobial robots (CARs), an innovative fusion of chemical and mechanical methodologies (Fig. 4D). These CARs utilize the chemical potency of iron oxide nanoparticles combined with their mechanical disruption abilities to attack both the structural and biological defenses of biofilms. The magneto-catalytic capabilities of these robots are driven by iron oxide nanoparticles that mimic POD activity, catalyzing H2O2 into reactive molecules that dismantle biofilms. Additionally, these nanoparticles, whether free-floating or encapsulated within various matrices, can be magnetically maneuvered to precisely target biofilm accumulations. The robots navigate predetermined paths to dislodge, scrub away, and eliminate bacterial remnants and biofilm residues.35 Thus, CARs offer a comprehensive approach to overcoming biofilm-associated infections in endodontic treatments.
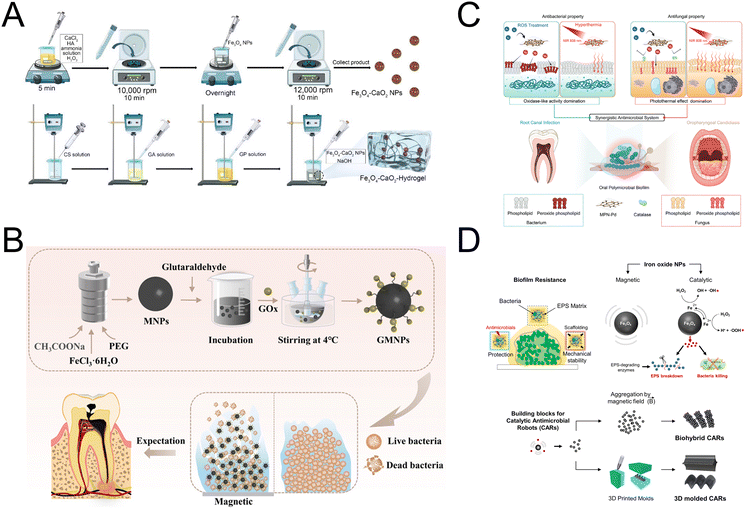 | ||
| Fig. 4 (A) Schematic diagram of the preparation of Fe3O4–CaO2 NPs and Fe3O4–CaO2-Hydrogel. Reproduced with permission from ref. 31. Copyright 2022, Multidisciplinary Digital Publishing Institute. (B) Schematic diagram of the preparation and application of MNPs and GMNPs. Reproduced with permission from ref. 32. Copyright 2021, American Chemical Society. (C) Schematic diagram of the MPN-Pd-mediated synergistic antimicrobial system for treating oral polymicrobial biofilm-associated infections. Reproduced with permission from ref. 34. Copyright 2023, John Wiley and Sons. (D) Catalytic and magnetic iron oxide NPs as building blocks for small-scale robots designed for biofilm killing and removal. Reproduced with permission from ref. 35. Copyright 2019, The American Association for the Advancement of Science. | ||
4.3 Periodontal disease
Periodontal disease, a common inflammatory disorder, is primarily caused by chronic bacterial infection from periodontal pathogens.85 It can result in tooth loss, affecting aesthetics, masticatory function, and overall quality of life, and may also be linked to systemic conditions such as diabetes, cardiovascular disease, and Alzheimer's disease.86 Effective management and timely removal of harmful oral microorganisms are essential for maintaining oral and overall health. Adjunctive antibiotic therapy with mechanical debridement is commonly used in periodontitis treatment to eliminate pathogenic microorganisms and reduce bacterial recolonization.87 However, the rise of bacterial resistance due to antibiotic abuse has created an urgent need for alternative strategies.88 Enzyme mimics have become a novel class of antibiotics, noted for their outstanding antibacterial properties, minimal systemic toxicity, and resistance to multi-drug resistance mechanisms.89Among novel materials for medical applications, nanodiamonds (NDs) and various carbon-based nanomaterials stand out due to their unique properties and functionalities.90,91 Fang et al. synthesized oxygenated nanodiamonds (O-NDs) with POD-like activity, capable of catalyzing the production of free radicals in the presence of low concentrations of H2O2 (Fig. 5A). These radicals enhance the destruction of bacterial cell membranes and biofilms, optimize periodontal inflammation management, and accelerate healing at periodontal infection sites.36 Further innovations in carbon-based nanomaterials include the work of Wu et al., who developed an injectable anti-biofilm ointment.37 This formulation combines Pt nanoparticle clusters (PtNCs) and graphitic carbon nitride (CN) with a mixture of PEG400/PEG4000. The CN-PtNC ointment exhibits both OXD-like and POD-like properties, enabling ROS production without the need for light, due to its efficient oxygen adsorption and activation capabilities. Notably, this ointment effectively treated periodontitis in rats, reducing inflammation and minimizing bone loss.37 To enhance the utilization of H2O2 by nanozymes, Shen et al. created FeSN nanozymes that effectively mimic active site of POD, achieving this through the self-assembled coordination of cysteine, histidine, and iron ions, resulting in high catalytic efficiency (Fig. 5B). The FeSN nanozyme displays distinctive antibacterial properties, causing an increase in ROS levels and a decrease in glutathione and ATP within F. nucleatum cells, which enhances the efficiency of bacterial eradication.38 Besides, Au nanoclusters (Au NCs) have also been recognized for their intrinsic enzyme-like activities, which include high catalytic efficiency and superior biocompatibility suitable for in vivo applications. However, the limited POD-like activity of Au NCs significantly hindered their use in antibacterial therapies.92 To overcome this, Wang et al. addressed the limitation by creating bimetallic nanoclusters, incorporating Pt atoms into Au NCs to improve the catalytic sites of the clusterzyme. Additionally, they developed a cascade catalytic nanozyme by chemically coupling GOx onto Au/Pt NCs, which transforms non-toxic glucose into gluconic acid and H2O2. This configuration optimizes the environment and substrate for POD reactions, enhancing antibacterial and antibiofilm activity against F. nucleatum.39
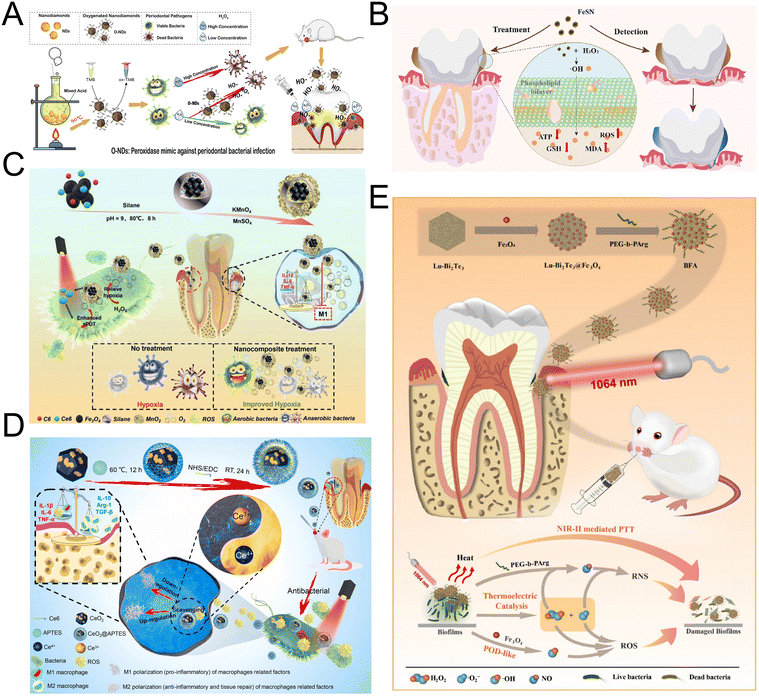 | ||
| Fig. 5 (A) Schematic diagram for the synthesis of O-NDs and the O-NDs/H2O2 system for antibacterial defense in periodontal diseases. Reproduced with permission from ref. 36. Copyright 2020, Elsevier. (B) Schematic diagram of enhanced bioactivity and caries-protective effects against biofilms using laboratory and in vivo models by co-delivering fluoride, iron, and tin on the outer enamel surface. Reproduced with permission from ref. 38. Copyright 2023, Elsevier. (C) Schematic diagram of the synthesis of F@Ce6-M NCs and their application in enhanced and selective antibacterial properties and downregulation of the pro-inflammatory cytokines for the treatment of periodontal diseases by ameliorating hypoxia. Reproduced with permission from ref. 40. Copyright 2021, John Wiley and Sons. (D) Schematic illustration of CeO2@Ce6 nanocomposite in synthesis, the antibacterial mechanism and modulating the polarization of macrophages for the treatment of periodontal diseases. Reproduced with permission from ref. 42. Copyright 2021, Elsevier. (E) Preparation and biomedical application of BFA. Reproduced with permission from ref. 43. Copyright 2023, Elsevier. | ||
Antimicrobial photodynamic therapy (aPDT) employs light-activated photosensitizers to generate cytotoxic ROS, offering a promising sterilization method.93,94 However, its effectiveness is compromised in hypoxic conditions, typical of periodontal disease, where anaerobic bacteria thrive and oxygen scarcity leads to less ROS generation and increased inflammation.95,96 Addressing this challenge, Sun et al. developed a nanoplatform that features MnO2-coated, amphiphilic silane-modified nanoparticles with an Fe3O4 core, Chlorin e6, and Coumarin 6 (Fig. 5C). This innovative design enhances aPDT efficacy by catalyzing H2O2 into O2, improving oxygen availability. It also supports magnetic targeting and real-time treatment monitoring, potentially overcoming hypoxia limitations in periodontal therapy.40 Building on this innovation, Santos et al. introduced another novel solution addressing the oxygen scarcity in periodontal treatments through the use of electrospinning technology. They developed composite fibrous membranes with a bead-on-string structure that effectively function as a controlled oxygen-release system. These membranes contain CaO2 nanoparticles as an oxygen-generating precursor and MnO2 nanosheets as nanozymes to catalyze H2O2 decomposition into oxygen.41 This approach not only complements the earlier advancements by providing a continuous oxygen supply but also significantly enhances the potential for periodontal healing by maintaining an oxygen-rich environment throughout the treatment process. However, aPDT can also precipitate pro-inflammatory effects due to excessive ROS, which disrupts the oxidant/antioxidant balance and attracts inflammatory cells, potentially damaging periodontal tissues.97 To address these inflammatory challenges, Sun et al. further innovated by developing CeO2@Ce6 nanocomposites that not only possess antibacterial properties but also mitigate inflammation (Fig. 5D). CeO2 NPs mimic SOD and CAT by catalytically reacting with superoxide and H2O2 through redox cycling between Ce3+ and Ce4+ ions. Under red light excitation at 630 nm, the CeO2@Ce6 nanocomposites achieve effective sterilization by enhancing ROS production during aPDT, followed by a swift reduction in ROS levels post-therapy due to the exceptional ROS-scavenging capacity of the CeO2. This dual function—boosting ROS for bacterial eradication and then curbing it to prevent inflammation—highlights the potential of CeO2@Ce6 nanocomposites to balance the therapeutic and inflammatory responses in periodontal disease treatment.42 In contrast, PTT operates on a different principle, utilizing photothermal agents to convert light into heat, effectively eliminating bacteria through hyperthermia. This heat disrupts cell membranes and denatures proteins, directly targeting free bacteria and biofilms.98 Expanding on this technique, Dai et al. introduced a synergistic approach that combines PTT with heat-induced ROS and RNS to enhance biofilm eradication and aid in the infected tissue recovery (Fig. 5E). Their innovative platform involves decorating lutetium-doped Bi2Te3 nanoplates with POD-like Fe3O4 and PEG-b-PArg. These nanoparticles catalyze reactions with H2O2 to produce highly reactive ˙OH, which then interacts with ˙O2− and NO to generate the potent RNS, ONOO−. The RNS and ROS generated are broad-spectrum antibacterial agents, causing nitrosative stress on biomacromolecules and damaging bacterial cell membranes and DNA.43
4.4 Peri-implantitis
Dental implants have become the leading clinical method for restoring the structure and function of missing teeth over the past four decades.99 Despite their high survival rate, the incidence of peri-implant diseases continues to rise.100 Mechanical decontamination methods can severely alter the implant's microstructure and surface electrochemical properties,101,102 while nonmechanical antiseptics often fail to remove tightly bound extracellular polymeric substance structures effectively.44 Systemic antibiotic administration has traditionally been preferred for treating implant-associated infections, but it frequently leads to antibiotic resistance.103 In response to these challenges, nanozymes have garnered considerable interest for their ability to impart antibacterial and anti-inflammatory properties to implant surfaces.3,104 These nanozymes catalyze H2O2 decomposition, generating ROS that destroy bacterial DNA, proteins, and lipids, thus mitigating bacterial resistance.105 This innovative approach promises to enhance antimicrobial efficacy while preserving the structural and functional integrity of dental implants, presenting a promising alternative to conventional treatments.Lee et al. devised a novel and safe treatment method for peri-implantitis, utilizing the dynamic action of micro-sized oxygen bubbles (Fig. 6A). These bubbles are produced from a catalytic reaction involving H2O2 and MnO2 nanozyme-doped silica diatom microparticles, referred to as diatom microbubblers (DM). The swift movement of these tiny DM particles allows them to navigate through the small spaces between implant screws, delivering just enough force to thoroughly eliminate biofilms without damaging the surrounding tissues or the surfaces of the implants.44 In deep tissues, the catalytic ROS production by nanozymes is often reduced due to restricted substrate diffusion. Therefore, it is crucial for nanozymes to have enhanced activity to generate sufficient ROS at inhibitory levels, even under low substrate concentrations, when addressing deep infections. Metal–organic frameworks (MOFs) are porous crystalline materials made of organic ligands and metal ions or clusters, notable for their ordered pore structures and large surface areas.106,107 Zhang et al. developed cerium-based metal–organic framework (Ce-BTC) coatings on medical titanium surfaces through a solvothermal process, followed by hydrogen plasma immersion ion implantation. These coatings feature numerous coordinatively unsaturated metal sites (Fig. 6B).45 The resulting Ce-BTC coatings exhibit a robust ATP deprivation capacity and OXD-like activity. This combination effectively inhibits biofilm formation and eradicates bacteria, especially in the acidic microenvironment induced by bacterial activity. Compared to conventional noble-metal or transition-metal oxide/sulfide-based nanozymes, single-atom nanozymes (SAzymes) feature atomically dispersed metal atoms that maximize atom utilization efficiency, significantly boosting their enzyme-like activities.108 Bai et al. developed a copper and silk fibroin complex to create copper SAzymes with atomically dispersed copper sites on ultrathin 2D porous N-doped carbon nanosheets (CuNx-CNS) (Fig. 6C). These SAzymes demonstrate POD, CAT, and OXD activities, effectively converting H2O2 and O2 into ROS via parallel and cascaded reactions. In vitro and in vivo experiments show that the optimized CuN4-CNS effectively inhibits multidrug-resistant bacteria and eradicates persistent biofilms, presenting significant therapeutic potential for deep implant-related biofilm infections.46 However, excessive inflammation can damage tissues surrounding the implant post-biofilm removal. Therefore, it is crucial to develop therapeutic agents that can modulate the inflammatory response throughout the various stages of treatment. To address this, Yang et al. prepared hollow Cu2MoS4 nanospheres (H-CMS NSs) with pH-responsive enzyme-like activities using an etching-precipitation method (Fig. 6D). These H-CMS NSs, with OXD/POD-like activities, generate ROS specifically in the acidic microenvironment of biofilms. In a neutral environment post-biofilm elimination, H-CMS NSs demonstrate CAT-like activities that inhibit M1 macrophage polarization and decrease proinflammatory cytokines. Yang's team has developed pH-responsive nanozymes capable of adaptively targeting biofilms while also modulating the macrophage-mediated inflammatory response, offering an efficient approach for treating implant infections.47
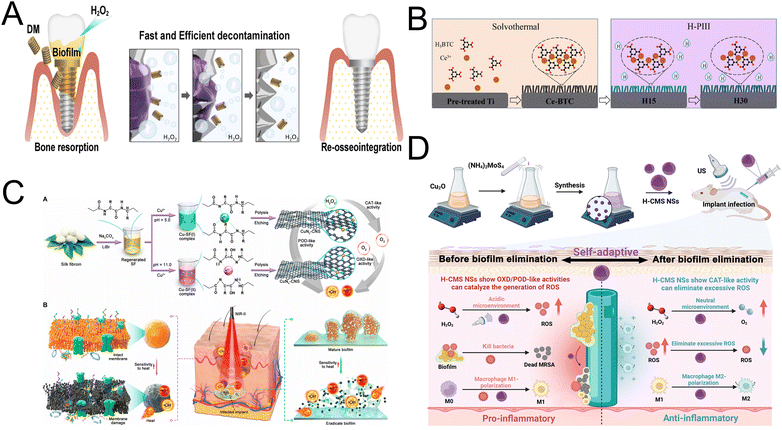 | ||
| Fig. 6 (A) Hypothetical schematic diagram of DM decontaminating the peri-implantitis-affected implant. Reproduced with permission from ref. 44. Copyright 2022, American Chemical Society. (B) Schematic diagram of the preparation of Ce-BTC, H15, and H30. Reproduced with permission from ref. 45. Copyright 2022, Elsevier. (C) Synthesis procedure and antibacterial therapy mechanism of CuNx-CNS SAzyme. Reproduced with permission from ref. 46. Copyright 2023, American Association for the Advancement of Science. (D) Preparing Hollow Cu2MoS4 Nanospheres with a self-adaptive antibiofilm effect and immune modulation for treating implant infections. Reproduced with permission from ref. 47. Copyright 2023, American Chemical Society. | ||
5. Challenges and perspectives
This review explores the classification, mechanisms, and dental applications of nanozymes, highlighting recent advances in antibacterial research that underscore their significant promise in dental care. The oral microbial community is closely related to both oral and systemic health.109 It can lead to dental caries, apical periodontitis, periodontal disease, pericoronitis, and oral mucosal disorders, and other oral conditions, as well as contribute to many systemic diseases.110 Natural enzymes such as proteolytic and amylase, recognized for their antibacterial, anti-inflammatory, and immune-enhancing properties, have been utilized to treat periodontitis, oral ulcers, and dental caries. However, these enzymes face significant challenges, including poor stability, high costs, labor-intensive purification, and difficulties in long-term storage.111 Advances in biomaterials, particularly nanozymes, have revolutionized oral health care by enhancing functionality and quality of life. Nanozymes, emerging as innovative alternatives, offer stability, scalability, and tunability.112 They provide unique solutions to oral infectious diseases through their distinct physicochemical properties and functional advantages, exhibiting antibacterial, antioxidant, and anti-inflammatory effects.113,114 Despite these promising advances, the full potential of nanozymes remains underutilized in dentistry. Many benefits observed in other medical fields have yet to be explored in dental applications, and numerous challenges highlighted in existing studies remain unresolved.Firstly, the mechanisms of nanozymes are not well-defined, and their catalytic efficiency is insufficient. Although some studies have proposed potential catalytic mechanisms, their precise workings remain unclear.115 Collaborative efforts in computational simulation, theoretical calculation, and artificial intelligence are crucial for advancing knowledge of nanozyme functions, enhancing their catalytic activities, and expanding their potential applications.116–118 Furthermore, the catalytic activity of nanozymes is currently inferior to that of natural enzymes, limiting their effectiveness in in vivo antibacterial applications. Recent studies suggest that the catalytic performance of nanozymes is influenced by their intrinsic physicochemical properties (shape, size, surface modifications) and external factors (temperature, pH, substrate concentration).119 The influence of the complex biological microenvironment on nanozyme activity and their long-term effects remains under explored. There is a pressing need to develop nanozymes with high catalytic efficiency that are well-suited for biological systems. Notably, SAzymes, which feature atomically dispersed active sites akin to those in natural metalloenzymes, represent a new frontier in cost-effective catalysis.120
Secondly, nanozymes exhibit limited specificity. In dentistry, nanozymes exhibit enzyme-like activities, including OXD, POD, CAT, and SOD. However, unlike natural enzymes, nanozymes often lack complex substrate-binding pockets, leading to nonspecific substrate interactions and behavior similar to conventional catalysts, which can cause side effects in biological settings.14,17 Thus, developing precise nanozyme-based therapies is a pressing research focus. Efforts should prioritize enhancing nanozyme selectivity for targeted therapies. Current research indicates that specificity may be enhanced by modifying nanozymes with aptamers, chiral molecules, or molecularly imprinted polymers, or by integrating them with natural enzymes that have inherent substrate selectivity.11 Future studies should focus on developing new types of nanozymes with increased specificity using these approaches, potentially leading to precise therapies that effectively treat specific dental conditions without adverse effects.
Thirdly, research on the antibacterial effects of nanozymes in dentistry lags behind their advancements in other areas of clinical medicine. While nanomaterials have addressed various therapeutic needs in dentistry, other medical fields have more thoroughly exploited the intrinsic properties of nanozymes—including light sensitivity, photothermal effects, magnetism, and synergistic chemodynamic properties—to enhance their catalytic activity.121,122 The emerging concept of nanocatalytic medicine, driven by extensive research, holds promise for monitoring, antibacterial treatments, tumor therapy, regeneration, and tissue protection.123–126 However, these innovations have yet to be widely applied in dentistry. By integrating the unique properties of nanozymes and leveraging their proven successes in other medical domains, substantial progress in dental treatments and patient outcomes could be achieved.
Despite the demonstrated efficacy of nanozymes in inhibiting bacterial growth in vitro, translating this innovative approach into clinical practice presents significant challenges due to the limited scope of current preclinical research. One of the primary challenges is the complexity of the oral cavity's microbial environment and dynamic conditions, which make it difficult to predict and control the effects of nanozymes on both pathogenic and beneficial microbes, as well as on host cells. Future studies should investigate the antibacterial activity of nanozymes and their unintended impacts on beneficial microbial communities, and develop nanozymes that can selectively target pathogenic bacteria while sparing beneficial microbes—key to maintaining oral health. Additionally, the unique interactions between nanozymes and host cells, such as their ability to achieve targeted intracellular delivery and controlled catalytic activity and magnetic field-driven drug release, represent a promising direction for future development.127,128 Therefore, carefully designed studies focusing on cellular uptake, intracellular degradation, and effects on cell viability are essential to assess the therapeutic potential and biocompatibility of nanozymes. Furthermore, evaluating potential nanozyme-induced inflammatory responses, alterations in immune signalling, and unintended cytotoxicity through comprehensive in vitro and in vivo immune assays is crucial to ensure their safety and understand their interactions with immune cells in clinical applications. Another primary challenge is the unclear nature of nanozymes' degradation products and their potential impacts on the body. Nanozymes often contain non-essential metal elements, whose accumulation in tissues can pose health risks.129,130 Strategies such as coating nanozymes with biocompatible polymers, using core–shell structures, and employing surface passivation, biocompatible coatings, and doping techniques can help mitigate adverse effects and enhance clinical viability. Furthermore, even at low concentrations, pH-dependent nanozymes can react strongly in gastric acid, potentially increasing the gastrointestinal burden, leading to weight loss, and elevating oxidative stress in blood and liver.131–133 Nanozymes' ability to be engineered for selective degradation or stability based on environmental triggers offers more precise control than traditional antimicrobials. Developing pH-responsive coatings or encapsulating materials could ensure their stability in acidic stomach environments, presenting a promising solution. Researchers should also design in vitro saliva simulation experiments to explore how natural enzymes affect nanozyme stability and catalytic activity, and develop appropriate animal models to study the distribution, accumulation, and clearance of nanozymes in vivo. This will help assess the potential impact of long-term enzyme interactions on nanozyme functionality loss or the emergence of toxic degradation products. In conclusion, although nanozymes offer an innovative and promising approach for managing oral infections, considerable efforts are needed to fully understand their interactions with the oral microbiome, ensure their long-term safety, address potential side effects, and determine their clinical applicability.
6. Conclusions
Antibacterial nanozymes have developed rapidly and hold significant potential for preventing and treating dental infectious diseases. However, they have not yet fully met clinical demands in dentistry, presenting numerous challenges and opportunities for further research and application. Unresolved issues require deeper exploration to understand their catalytic mechanisms and to develop new nanozyme varieties suitable for clinical trials. It is essential for dental researchers to collaborate on molecular studies, address specific clinical challenges, and evaluate the long-term effects of nanozymes in the oral environment. This review aims to spark interest and provide insights into the antibacterial properties of nanozymes, advocating for continued research to develop safe and effective nanozymes for future clinical applications.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Lipeng Liu: conceptualization, investigation, writing – original draft, writing – review & editing. Yaoyuan Zhang: investigation, methodology, writing – original draft. Tianjuan Ju: writing – original draft. Xutao Chen: conceptualization, methodology. Xinwei Li: visualization. Li-an Wu: conceptualization, supervision, project administration, funding acquisition, writing – review & editing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82370986), Shaanxi Provincial Health Research Innovation Team Project (Grant No. 2023TD-01), the Project Supported by Natural Science Basic Research Plan in Shaanxi Province of China (Grant No. 2024JC-YBQN-0808) and the Xi'an Municipal Science and Technology Project (Grant No. 2024JH-YLYB-0465).Notes and references
- M. A. Peres, L. M. D. Macpherson, R. J. Weyant, B. Daly, R. Venturelli, M. R. Mathur, S. Listl, R. K. Celeste, C. C. Guarnizo-Herreno, C. Kearns, H. Benzian, P. Allison and R. G. Watt, Lancet, 2019, 394, 249–260 CrossRef PubMed.
- Y.-H. Lee, H.-W. Park, J.-H. Lee, H.-W. Seo and S.-Y. Lee, Int. J. Oral Sci., 2012, 4, 196–201 CrossRef CAS PubMed.
- S. Hosseinpour, A. Nanda, L. J. Walsh and C. Xu, Nanomaterials, 2021, 11, 2236 CrossRef PubMed.
- A. Pulcini, J. Bollaín, I. Sanz-Sánchez, E. Figuero, B. Alonso, M. Sanz and D. Herrera, J. Clin. Periodontol., 2019, 46, 342–353 CrossRef CAS PubMed.
- J. van der Heijden, L. A. Reynolds, W. Deng, A. Mills, R. Scholz, K. Imami, L. J. Foster, F. Duong and B. B. Finlay, mBio, 2016, 7, e01238 CrossRef CAS PubMed.
- T. Li, N. Wang, S. Chen, R. Lu, H. Li and Z. Zhang, Int. J. Nanomed., 2017, 12, 2995–3007 CrossRef CAS PubMed.
- S. Daly, J. Seong, R. Newcombe, M. Davies, J. Nicholson, M. Edwards and N. West, J. Dent., 2019, 80(Suppl 1), S26–s32 CrossRef CAS PubMed.
- X. Wang, W. Cao, L. Qin, T. Lin, W. Chen, S. Lin, J. Yao, X. Zhao, M. Zhou, C. Hang and H. Wei, Theranostics, 2017, 7, 2277–2286 CrossRef CAS PubMed.
- X. Zhang, X. Chen and Y. Zhao, Nano-Micro Lett., 2022, 14, 95 CrossRef CAS PubMed.
- X. Ren, D. Chen, Y. Wang, H. Li, Y. Zhang, H. Chen, X. Li and M. Huo, J. Nanobiotechnol., 2022, 20, 92 CrossRef CAS PubMed.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- M. Liang and X. Yan, Acc. Chem. Res., 2019, 52, 2190–2200 CrossRef CAS PubMed.
- X. Chen, H. Xing, Z. Zhou, Y. Hao, X. Zhang, F. Qi, J. Zhao, L. Gao and X. Wang, J. Mater. Chem. B, 2021, 9, 1491–1502 RSC.
- Y. Cai, Y. Li, J. Zhang, N. Tang, X. Bao and Z. Liu, Particuology, 2023, 80, 61–73 CrossRef CAS.
- Y. Zhang, G. Wei, W. Liu, T. Li, Y. Wang, M. Zhou, Y. Liu, X. Wang and H. Wei, Nat. Rev. Methods Primers, 2024, 4, 36 CrossRef CAS.
- L. Gao, Y. Liu, D. Kim, Y. Li, G. Hwang, P. C. Naha, D. P. Cormode and H. Koo, Biomaterials, 2016, 101, 272–284 CrossRef CAS PubMed.
- Y. Liu, P. C. Naha, G. Hwang, D. Kim, Y. Huang, A. Simon-Soro, H. I. Jung, Z. Ren, Y. Li, S. Gubara, F. Alawi, D. Zero, A. T. Hara, D. P. Cormode and H. Koo, Nat. Commun., 2018, 9, 2920 CrossRef PubMed.
- Y. Liu, Y. Huang, D. Kim, Z. Ren, M. J. Oh, D. P. Cormode, A. T. Hara, D. T. Zero and H. Koo, Nano Lett., 2021, 21, 9442–9449 CrossRef CAS PubMed.
- Y. Huang, Y. Liu, N. K. Pandey, S. Shah, A. Simon-Soro, J. C. Hsu, Z. Ren, Z. Xiang, D. Kim, T. Ito, M. J. Oh, C. Buckley, F. Alawi, Y. Li, P. J. M. Smeets, S. Boyer, X. Zhao, D. Joester, D. T. Zero, D. P. Cormode and H. Koo, Nat. Commun., 2023, 14, 6087 CrossRef CAS PubMed.
- J. Liao, L. Zhang, B. Sun, D. Wang, Z. Zhang, W. Ma, Z. Wang, Y. Wang, Q. Wang, W. Yin and Z. Gu, Nano Today, 2024, 55, 102204 CrossRef CAS.
- P. C. Naha, Y. Liu, G. Hwang, Y. Huang, S. Gubara, V. Jonnakuti, A. Simon-Soro, D. Kim, L. Gao, H. Koo and D. P. Cormode, ACS Nano, 2019, 13, 4960–4971 CrossRef CAS PubMed.
- Y. Huang, Y. Liu, S. Shah, D. Kim, A. Simon-Soro, T. Ito, M. Hajfathalian, Y. Li, J. C. Hsu, L. M. Nieves, F. Alawi, P. C. Naha, D. P. Cormode and H. Koo, Biomaterials, 2021, 268, 120581 CrossRef CAS PubMed.
- Q. Dong, Z. Li, J. Xu, Q. Yuan, L. Chen and Z. Chen, Nano Res., 2022, 15, 9800–9808 CrossRef CAS.
- Y. Wang, X. Shen, S. Ma, Q. Guo, W. Zhang, L. Cheng, L. Ding, Z. Xu, J. Jiang and L. Gao, Biomater. Sci., 2020, 8, 2447–2458 RSC.
- J. Guo, M. D. Liu, W. Lei, Y. Xu, K. Li, J. Yu, Y. X. Sun, C. Huang and X. Z. Zhang, Adv. Funct. Mater., 2023, 33, 2213729 CrossRef CAS.
- S. Bukhari, D. Kim, Y. Liu, B. Karabucak and H. Koo, J. Endod., 2018, 44, 806–812 CrossRef PubMed.
- J. Song, L. Hong, X. Zou, H. Alshawwa, Y. Zhao, H. Zhao, X. Liu, C. Si and Z. Zhang, Int. J. Mol. Sci., 2022, 23, 10107 CrossRef CAS PubMed.
- Y. Ji, Z. Han, H. Ding, X. Xu, D. Wang, Y. Zhu, F. An, S. Tang, H. Zhang, J. Deng and Q. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 17289–17299 CrossRef CAS PubMed.
- T. Aslan, S. Dadi, O. Kafdag, N. Temur, N. Ildiz, I. Ocsoy and Y. Ustun, Odontology, 2024, 112, 444–452 CrossRef CAS PubMed.
- L. Chen, M. Peng, H. Li, J. Zhou, W. He, R. Hu, F. Ye, Y. Li, L. Shi and Y. Liu, Adv. Mater., 2024, 36, 2306376 CrossRef CAS PubMed.
- G. Hwang, A. J. Paula, E. E. Hunter, Y. Liu, A. Babeer, B. Karabucak, K. Stebe, V. Kumar, E. Steager and H. Koo, Sci. Robot., 2019, 4, eaaw2388 CrossRef PubMed.
- J. Fang, H. Wang, X. Bao, Y. Ni, Y. Teng, J. Liu, X. Sun, Y. Sun, H. Li and Y. Zhou, Carbon, 2020, 169, 370–381 CrossRef CAS.
- T. Wu, J. Sun, J. Lei, Q. Fan, X. Tang, G. Zhu, Q. Yan, X. Feng and B. Shi, Nanoscale, 2021, 13, 17912–17919 RSC.
- B. Shen, L. Yang, H. Xu, Y. Zhang, D. Ming, L. Zhu, Y. Wang and L. Jiang, J. Colloid Interface Sci., 2023, 650, 211–221 CrossRef CAS PubMed.
- Y. Wang, C. Li, B. Shen, L. Zhu, Y. Zhang and L. Jiang, Chem. Eng. J., 2023, 466, 143292 CrossRef CAS.
- X. Sun, J. Sun, Y. Sun, C. Li, J. Fang, T. Zhang, Y. Wan, L. Xu, Y. Zhou, L. Wang and B. Dong, Adv. Funct. Mater., 2021, 31, 2101040 CrossRef CAS.
- D. M. dos Santos, L. M. Dias, A. K. Surur, D. A. de Moraes, A. C. Pavarina, C. R. Fontana and D. S. Correa, ACS Appl. Nano Mater., 2022, 5, 14425–14436 CrossRef CAS.
- Y. Sun, X. Sun, X. Li, W. Li, C. Li, Y. Zhou, L. Wang and B. Dong, Biomaterials, 2021, 268, 120614 CrossRef CAS PubMed.
- X. Dai, Y. Liu, F. Meng, Q. Li, F. Wu, J. Yuan, H. Chen, H. Lv, Y. Zhou and Y. Chang, Acta Biomater., 2023, 171, 519–531 CrossRef CAS PubMed.
- E. H. Lee, S. W. Lee, Y. Seo, Y. H. Deng, Y. J. Lim, H. B. Kwon, K. Park, H. Kong and M. J. Kim, ACS Appl. Mater. Interfaces, 2022, 14, 27634–27650 CrossRef CAS PubMed.
- H. Zhang, J. Qiu, M. Xing, X. Liu, X. Ma, L. Ouyang, Y. Qiao, W. Qian and X. Liu, Chem. Eng. J., 2022, 449, 137881 CrossRef CAS.
- J. Bai, Y. Feng, W. Li, Z. Cheng, J. M. Rosenholm, H. Yang, G. Pan, H. Zhang and D. Geng, Research, 2023, 6, 0031 CrossRef CAS PubMed.
- K. Yang, H. Dong, W. Xiu, L. Yuwen, Y. Mou, Z. Yin, B. Liang and L. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 18720–18733 CrossRef CAS PubMed.
- J. Jin, W. Song, J. Wang, L. Li, Y. Tian, S. Zhu, Y. Zhang, S. Xu, B. Yang and B. Zhao, Chem. Eng. J., 2022, 430, 132687 CrossRef CAS.
- X. Yang, J. Xiang, W. Su, J. Guo, J. Deng, L. Tang, G. Li, Y. Liang, L. Zheng, M. He, J. Zhong and J. Zhao, Nano Today, 2023, 49, 101809 CrossRef CAS.
- F. Wei, X. Cui, Z. Wang, C. Dong, J. Li and X. Han, Chem. Eng. J., 2021, 408, 127240 CrossRef CAS PubMed.
- X. Liu, L. Yan, H. Ren, Y. Cai, C. Liu, L. Zeng, J. Guo and A. Liu, Biosens. Bioelectron., 2020, 165, 112342 CrossRef CAS PubMed.
- Y. Ye, J. Zou, W. Wu, Z. Wang, S. Wen, Z. Liang, S. Liu, Y. Lin, X. Chen, T. Luo, L. Yang, Q. Jiang and L. Guo, Nanoscale, 2024, 16, 3324–3346 RSC.
- A. Sahu, J. Jeon, M. S. Lee, H. S. Yang and G. Tae, Mater. Sci. Eng., C, 2021, 119, 111596 CrossRef CAS PubMed.
- L. Mei, S. Zhu, Y. Liu, W. Yin, Z. Gu and Y. Zhao, Chem. Eng. J., 2021, 418, 129431 CrossRef CAS.
- Q. Liu, A. Zhang, R. Wang, Q. Zhang and D. Cui, Nanomicro Lett, 2021, 13, 154 CAS.
- X. Liu, H. Xu, H. Peng, L. Wan, D. Di, Z. Qin, L. He, J. Lu, S. Wang and Q. Zhao, Coord. Chem. Rev., 2024, 502, 215610 CrossRef CAS.
- Z. Liu, F. Wang, J. Ren and X. Qu, Biomaterials, 2019, 208, 21–31 CrossRef CAS PubMed.
- W. Sun, L. Feng, J. Zhang, K. Lin, H. Wang, B. Yan, T. Feng, M. Cao, T. Liu, Y. Yuan and N. Wang, Adv. Sci., 2022, 9, e2105008 CrossRef PubMed.
- H. Sies, V. V. Belousov, N. S. Chandel, M. J. Davies, D. P. Jones, G. E. Mann, M. P. Murphy, M. Yamamoto and C. Winterbourn, Nat. Rev. Mol. Cell Biol., 2022, 23, 499–515 CrossRef CAS PubMed.
- F. Attar, M. G. Shahpar, B. Rasti, M. Sharifi, A. A. Saboury, S. M. Rezayat and M. Falahati, J. Mol. Liq., 2019, 278, 130–144 CrossRef CAS.
- M. M. F. A. Baig, A. Fatima, X. Gao, A. Farid, M. Ajmal Khan, A. W. Zia and H. Wu, J. Controlled Release, 2022, 352, 98–120 CrossRef CAS PubMed.
- Z. Chen, Z. Wang, J. Ren and X. Qu, Acc. Chem. Res., 2018, 51, 789–799 CrossRef CAS PubMed.
- H. Kim, E. H. Lee, S. W. Lee, Y. H. Deng, H. B. Kwon, Y. J. Lim, H. Kong and M. J. Kim, BMC Oral Health, 2023, 23, 33 CrossRef CAS PubMed.
- J. L. Baker, B. Bor, M. Agnello, W. Shi and X. He, Trends Microbiol., 2017, 25, 362–374 CrossRef CAS PubMed.
- C. Moissl-Eichinger, M. Pausan, J. Taffner, G. Berg, C. Bang and R. A. Schmitz, Trends Microbiol., 2018, 26, 70–85 CrossRef CAS PubMed.
- W. H. Bowen, R. A. Burne, H. Wu and H. Koo, Trends Microbiol., 2018, 26, 229–242 CrossRef CAS PubMed.
- P. D. Marsh and E. Zaura, J. Clin. Periodontol., 2017, 44(Suppl 18), S12–S22 Search PubMed.
- Z. Liu, S. Ma, X. Lu, T. Zhang, Y. Sun, W. Feng, G. Zheng, L. Sui, X. Wu, X. Zhang and P. Gao, Chem. Eng. J., 2019, 356, 117–129 CrossRef CAS.
- R. J. Lamont, H. Koo and G. Hajishengallis, Nat. Rev. Microbiol., 2018, 16, 745–759 CrossRef CAS PubMed.
- C. M. Ardila and J. A. Bedoya-Garcia, Int. J. Dent. Hyg., 2023, 21, 141–148 CrossRef PubMed.
- Z. Chen, Z. Chu, Y. Jiang, L. Xu, H. Qian, Y. Wang and W. Wang, Mater. Today Bio, 2023, 20, 100635 CrossRef CAS PubMed.
- A. La Fontaine, A. Zavgorodniy, H. Liu, R. Zheng, M. Swain and J. Cairney, Sci. Adv., 2016, 2, e1601145 CrossRef PubMed.
- E. M. Decker, C. Klein, D. Schwindt and C. von Ohle, Int. J. Oral Sci., 2014, 6, 195–204 CrossRef CAS PubMed.
- W. H. Bowen, R. A. Burne, H. Wu and H. Koo, Trends Microbiol., 2018, 26, 229–242 CrossRef CAS PubMed.
- J. L. del Pozo and R. Patel, Clin. Pharmacol. Ther., 2007, 82, 204–209 CrossRef CAS PubMed.
- M. J. Noto, H. J. Domenico, D. W. Byrne, T. Talbot, T. W. Rice, G. R. Bernard and A. P. Wheeler, JAMA, 2015, 313, 369–378 CrossRef PubMed.
- S. Zanganeh, G. Hutter, R. Spitler, O. Lenkov, M. Mahmoudi, A. Shaw, J. S. Pajarinen, H. Nejadnik, S. Goodman, M. Moseley, L. M. Coussens and H. E. Daldrup-Link, Nat. Nanotechnol., 2016, 11, 986–994 CrossRef CAS PubMed.
- G. R. Germaine and C. F. Schachtele, Infect. Immun., 1976, 13, 365–372 CrossRef CAS PubMed.
- J. W. Choi and S. Y. Yang, Polymers, 2023, 15, 529 CrossRef CAS PubMed.
- J. Wong, D. Manoil, P. Nasman, G. N. Belibasakis and P. Neelakantan, Front. Oral Health, 2021, 2, 672887 CrossRef PubMed.
- P. N. Nair, Int. Endod. J., 2006, 39, 249–281 CrossRef CAS PubMed.
- R. Ordinola-Zapata, W. C. Noblett, A. Perez-Ron, Z. Ye and J. Vera, Int. Endod. J., 2022, 55(Suppl 3), 613–636 CrossRef PubMed.
- N. Raura, A. Garg, A. Arora and M. Roma, Biomater. Res., 2020, 24, 21 CrossRef PubMed.
- R. Ordinola-Zapata, C. M. Bramante, B. Cavenago, M. S. Graeff, I. Gomes de Moraes, M. Marciano and M. A. Duarte, Int. Eendod. J., 2012, 45, 162–168 CrossRef CAS PubMed.
- T.-J. Li, Y.-h. Hao, Y.-l. Tang and X.-h. Liang, Front. Microbiol., 2022, 13, 919633 CrossRef PubMed.
- T. T. T. Vo, P. M. Chu, V. P. Tuan, J. S. Te and I. T. Lee, Antioxidants, 2020, 9, 1211 CrossRef CAS PubMed.
- S. L. Munasur, E. B. Turawa, U. M. E. Chikte and A. Musekiwa, Int. J. Environ. Res. Public Health, 2020, 17, 5601 CrossRef PubMed.
- J. Pulit-Prociak, A. Staroń, P. Staroń, A. Chmielowiec-Korzeniowska, A. Drabik, L. Tymczyna and M. Banach, J. Nanobiotechnol., 2020, 18, 148 CrossRef CAS PubMed.
- C. Zhou, Q. Wang, J. Jiang and L. Gao, Antibiotics, 2022, 11, 390 CrossRef CAS PubMed.
- J.-X. Qin, X.-G. Yang, C.-F. Lv, Y.-Z. Li, K.-K. Liu, J.-H. Zang, X. Yang, L. Dong and C.-X. Shan, Mater. Des., 2021, 210, 110091 CrossRef CAS.
- N. Rao, R. Singh and L. Bashambu, Mater. Today: Proc., 2021, 44, 608–614 CAS.
- L. Hu, H. Liao, L. Feng, M. Wang and W. Fu, Anal. Chem., 2018, 90, 6247–6252 CrossRef CAS PubMed.
- M. He, Z. Wang, H. Yang, Q. Wang, D. Xiang, X. Pang, Y. K. Chan, D. Sun, G. Yin, W. Yang and Y. Deng, Adv. Sci., 2023, 10, e2300986 CrossRef PubMed.
- M. Piksa, C. Lian, I. C. Samuel, K. J. Pawlik, I. D. W. Samuel and K. Matczyszyn, Chem. Soc. Rev., 2023, 52, 1697–1722 RSC.
- R. T. Mendes, D. Nguyen, D. Stephens, F. Pamuk, D. Fernandes, H. Hasturk, T. E. Van Dyke and A. Kantarci, Clin. Exp. Dent. Res., 2018, 4, 241–248 CrossRef PubMed.
- D. Yang, G. Yang, S. Gai, F. He, C. Li and P. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 6829–6838 CrossRef CAS PubMed.
- X. Li, S. Ren, L. Song, D. Gu, H. Peng, Y. Zhao, C. Liu, J. Yang and L. Miao, Int. J. Nanomed., 2023, 18, 813–827 CrossRef CAS PubMed.
- J. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Y. Liang, Z. Li, S. Zhu, Y. Zheng, K. W. K. Yeung, X. Wang and S. Wu, Nat. Commun., 2019, 10, 4490 CrossRef PubMed.
- S. Wu, J. Xu, L. Zou, S. Luo, R. Yao, B. Zheng, G. Liang, D. Wu and Y. Li, Nat. Commun., 2021, 12, 3303 CrossRef CAS PubMed.
- G. E. Salvi, R. Cosgarea and A. Sculean, J. Dent. Res., 2017, 96, 31–37 CrossRef CAS PubMed.
- A. Mellado-Valero, P. Buitrago-Vera, M. F. Sola-Ruiz and J. C. Ferrer-Garcia, Med. Oral Patol. Oral Cir. Bucal, 2013, 18, e869–e876 CrossRef PubMed.
- J. Prathapachandran and N. Suresh, Dent Res. J., 2012, 9, 516–521 CrossRef PubMed.
- M. Esposito, M. G. Grusovin and H. V. Worthington, Cochrane Database Syst. Rev., 2013, 2013, Cd004152 Search PubMed.
- G. M. Esteves, J. Esteves, M. Resende, L. Mendes and A. S. Azevedo, Antibiotics, 2022, 11, 235 CrossRef CAS PubMed.
- Y. Dai, Y. Ding and L. Li, Chin. Chem. Lett., 2021, 32, 2715–2728 CrossRef CAS.
- Y. Huang, Q. Kou, Y. Su, L. Lu, X. Li, H. Jiang, R. Gui, R. Huang, X. Nie and J. Li, J. Nanobiotechnol., 2023, 21, 89 CrossRef CAS PubMed.
- L. Jiao, J. Y. R. Seow, W. S. Skinner, Z. U. Wang and H.-L. Jiang, Mater. Today, 2019, 27, 43–68 CrossRef CAS.
- L. Huang, J. Chen, L. Gan, J. Wang and S. Dong, Sci. Adv., 2019, 5, eaav5490 CrossRef CAS PubMed.
- J. L. Baker, J. L. Mark Welch, K. M. Kauffman, J. S. McLean and X. He, Nat. Rev. Microbiol., 2024, 22, 89–104 CrossRef CAS PubMed.
- P. Xian, Z. Xuedong, X. Xin, L. Yuqing, L. Yan, L. Jiyao, S. Xiaoquan, H. Shi, X. Jian and L. Ga, Int. J. Oral Sci., 2018, 10, 16 CrossRef PubMed.
- M. Hosseini Hooshiar, A. Badkoobeh, S. Kolahdouz, A. Tadayonfard, A. Mozaffari, K. Nasiri, S. Salari, R. Safaralizadeh and S. Yasamineh, J. Nanobiotechnol., 2024, 22, 207 CrossRef CAS PubMed.
- Y. Chen, H. Zou, B. Yan, X. Wu, W. Cao, Y. Qian, L. Zheng and G. Yang, Adv. Sci., 2022, 9, e2103977 CrossRef PubMed.
- K. Yang, H. Dong, W. Xiu, L. Yuwen, Y. Mou, Z. Yin, B. Liang and L. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 18720–18733 CrossRef CAS PubMed.
- B. Zhu, J. Wu, T. Li, S. Liu, J. Guo, Y. Yu, X. Qiu, Y. Zhao, H. Peng, J. Zhang, L. Miao and H. Wei, Adv. Healthc. Mater., 2024, 13, e2302485 CrossRef PubMed.
- L. Gao and X. Yan, Sci. China Life Sci., 2016, 59, 400–402 CrossRef PubMed.
- C. Du, W. Feng, X. Dai, J. Wang, D. Geng, X. Li, Y. Chen and J. Zhang, Small, 2022, 18, e2203031 CrossRef PubMed.
- X. Zhu, H. Li, S. Hou, P. Song, J. Zheng, T. Wu, H. Zhao and Q. Liu, Chem. Eng. J., 2024, 482, 148589 CrossRef CAS.
- Z. Chen, Y. Yu, Y. Gao and Z. Zhu, ACS Nano, 2023, 17, 13062–13080 CrossRef CAS PubMed.
- Z. Wang, R. Zhang, X. Yan and K. Fan, Mater. Today, 2020, 41, 81–119 CrossRef CAS.
- Y. Shi, Z. Ma, X. Zhang, Z. Ma, F. Yan, C. Zhu and Y. Chen, Adv. Funct. Mater., 2024, 34, 2403508 CrossRef CAS.
- X. Xiang, H. Pang, T. Ma, F. Du, L. Li, J. Huang, L. Ma and L. Qiu, J Nanobiotechnol., 2021, 19, 92 CrossRef CAS PubMed.
- F. Wang, E. Ju, Y. Guan, J. Ren and X. Qu, Small, 2017, 13, 1603051 CrossRef PubMed.
- C. Cao, N. Yang, X. Wang, J. Shao, X. Song, C. Liang, W. Wang and X. Dong, Coord. Chem. Rev., 2023, 491, 215245 CrossRef CAS.
- G. Sharma, S. Chatterjee, C. Chakraborty and J. C. Kim, Pharmacol. Rev., 2023, 75, 739–757 CrossRef CAS PubMed.
- J. Zhuang, A. C. Midgley, Y. Wei, Q. Liu, D. Kong and X. Huang, Adv. Mater., 2024, 36, 2210848 CrossRef CAS PubMed.
- J. Sheng, Y. Wu, H. Ding, K. Feng, Y. Shen, Y. Zhang and N. Gu, Adv. Mater., 2024, 36, 2211210 CrossRef CAS PubMed.
- D. Mehta and S. Singh, Int. J. Biol. Macromol., 2024, 278, 134582 CrossRef CAS PubMed.
- S. Ganguly, P. Das, S. Srinivasan, A. R. Rajabzadeh, X. S. Tang and S. Margel, ACS Appl. Nano Mater., 2024, 7, 5272–5286 CrossRef CAS.
- J. Schoon, B. Hesse, A. Rakow, M. J. Ort, A. Lagrange, D. Jacobi, A. Winter, K. Huesker, S. Reinke, M. Cotte, R. Tucoulou, U. Marx, C. Perka, G. N. Duda and S. Geissler, Adv. Sci., 2020, 7, 2000412 CrossRef CAS PubMed.
- B. Zhu, L. Li, B. Wang, L. Miao, J. Zhang and J. Wu, Chembiochem, 2023, 24, e202200636 CrossRef CAS PubMed.
- S. Shi, X. Ou and D. Cheng, Int. J. Nanomed., 2024, 19, 19–34 CrossRef CAS PubMed.
- A. Besinis, T. De Peralta, C. J. Tredwin and R. D. Handy, ACS Nano, 2015, 9, 2255–2289 CrossRef CAS PubMed.
- D. S. W. Benoit, K. R. Sims Jr and D. Fraser, ACS Nano, 2019, 13, 4869–4875 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |