From synthesis to chiroptical activities: advancements in circularly polarized luminescent inorganic quantum dots
Xinyu
Wang
,
Wenhui
Yan
,
Dai-Wen
Pang
 and
Jiarong
Cai
and
Jiarong
Cai
 *
*
State Key Laboratory of Medicinal Chemical Biology, Tianjin Key Laboratory of Biosensing and Molecular Recognition, Frontiers Science Center for New Organic Matter, Research Center for Analytical Sciences, College of Chemistry, Frontiers Science Center for Cell Responses, Engineering Research Center of Thin Film Optoelectronics Technology, Ministry of Education, School of Materials Science and Engineering, Smart Sensing Interdisciplinary Science Center, Haihe Laboratory of Sustainable Chemical Transformations, Nankai University, Tianjin, 300071, P. R. China. E-mail: jrcai@nankai.edu.cn
First published on 30th October 2024
Abstract
Circularly polarized luminescence (CPL) in inorganic quantum dots (QDs) represents a burgeoning and dynamic research domain, offering immense potential across a spectrum of applications, including three-dimensional displays, optical data storage, asymmetric catalysis, and chiral sensing. However, the persistent trade-off between fluorescence brightness and the emission dissymmetry factor highlights the nascent stage of current research. This review delves into the synthesis methodologies of CPL QDs, providing an exhaustive analysis of existing approaches and the resulting material properties. It elucidates the critical factors influencing CPL characteristics, such as ligand types, interaction modes, and QD architectures. Furthermore, it synthesizes the theoretical frameworks underlying chirality and CPL generation, ranging from time-dependent density functional theory (TDDFT) to ab initio molecular dynamics (AIMD), thereby deepening the understanding of CPL mechanisms within QDs. The review culminates with a comprehensive exploration of potential applications, alongside a forward-looking perspective on the future trajectory of CPL QD research.
1 Introduction
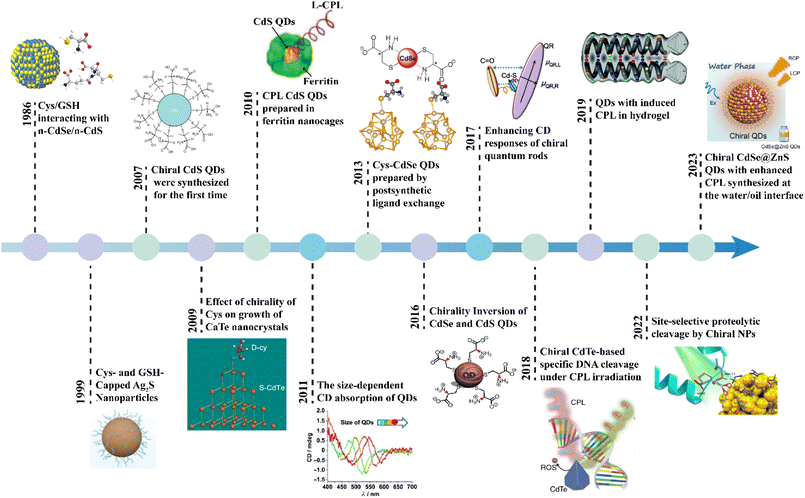
Circularly polarized luminescence (CPL) is defined by the preferential emission of left- or right-handed circularly polarized light by chiral luminescent materials in their excited states.1–3 This unique optical phenomenon has attracted considerable attention, owing to its expansive potential applications across a wide array of fields, including three-dimensional (3D) displays,4–6 optical storage,7 anti-counterfeiting,6,8,9 asymmetric synthesis,10–12 chiral sensing,13–16 information encryption,17,18 and quantum communications.19 A vast array of CPL-active materials has been meticulously designed and synthesized, encompassing organic molecules,20,21 lanthanide complexes,22,23 polymers,24 supramolecule architectures,25–27 inorganic substances,28,29 and chiral liquid crystals.30–32 In the present review, studies focusing on inorganic quantum dots (QDs) with chiral properties, a particularly exciting class of chiral nanomaterials, have been considered. QDs are luminescent semiconductor nanoparticles with outstanding tunability of the optical and physical properties as a result of their variable size dependent band electronic structure due to the quantum confinement effect. Due to these features, QDs have emerged as particularly promising candidates for CPL-related applications owing to their exceptional brightness and remarkable stability.33,34Tracing the evolution of CPL QDs, one sees a progression that, while relatively nascent as a distinct concept, has been in development for several decades (Fig. 1). In the 1990s, researchers frequently synthesized QDs using chiral cysteine (Cys) or glutathione (GSH), leveraging these chiral ligands as both reducing agents and stabilizing agents.35,36 At that time, the primary focus was on understanding how these ligands influenced the growth and luminescence properties of QDs, with little emphasis on their polarization characteristics. A pivotal moment in the field occurred in 2007, when Gun'ko's team synthesized chiral CdS QDs using chiral penicillamine (Pen) under microwave heating, marking a significant milestone and formally introducing the concept of chiral QDs.37 Subsequent research expanded the exploration of various chiral QDs, including CdSe,38,39 CdS,40 PbS,41 AgInS,42 CuInS2,43 Ag2S,44 and InP,45 across diverse morphologies such as spherical,46 rod-shaped,47,48 matchstick-like,49 and tetrapodal structures.50 These advances broadened the potential applications of chiral QDs to areas like chiral sensing,51,52 asymmetric catalysis,10,53 and biomedical applications.50,54 Advances in CPL measurement instrumentation have significantly deepened the understanding of and heightened interest in the CPL properties of materials.55,56 Notably, researchers achieved CPL QDs by encapsulating them in chiral ferritin nanocages, thereby formally linking the CPL concept with QDs.38 Since then, the field has expanded from single inorganic QDs to more complex self-assembled structures,57,58 and supramolecular architectures,59,60 with applications extending into anti-counterfeiting technologies and optical imaging.
The practical application of CPL properties in QDs presents significant challenges, with a persistent issue being the difficulty in balancing photoluminescence brightness with chiral luminescence asymmetry—two aspects that often seem intrinsically opposed.61,62 This imbalance has notably hindered the performance of CPL QDs, as evidenced by the current CPL inorganic QDs, which exhibit absolute photoluminescence quantum yield (PLQY) commonly below 40% and chiral luminescence dissymmetry factors ranging from 10−5 to 10−2.63 Addressing these challenges, this review offers a thorough, synthesis-centered analysis of CPL QDs, systematically detailing their preparation methods and their respective scope. Furthermore, it delves into signal modulation strategies within the synthesis process, providing crucial insights into the creation of high-quality CPL QDs and the fine-tuning of their optical properties. The review also examines the origins of chirality in CPL QDs and explores potential mechanisms underlying their CPL behavior. Ultimately, the discussion extends to practical applications, offering a forward-looking perspective on the future development and utilization of CPL QDs (Fig. 2).
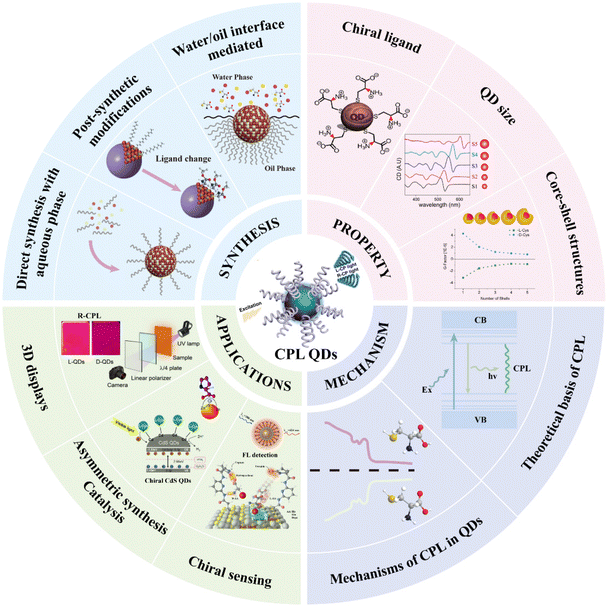 | ||
| Fig. 2 Schematic overview of the synthesis strategies, property modulation, luminescence mechanism and applications of CPL QDs. | ||
It is important to note that this review does not extensively cover carbon dots and perovskite QDs due to their distinct growth mechanisms and the availability of comprehensive reviews on these materials.28,64–66 Similarly, studies focusing on the acquisition of chirality through the self-assembly of QDs with chiral supramolecular structures are beyond the scope of this review.
2 Synthesis strategies
The preparation methods for CPL-active or chiral inorganic QDs can be categorized into three main pathways: direct aqueous-phase synthesis, post-synthesis modification, and water/oil interface-mediated synthesis. In this review, we provide a comprehensive overview of these approaches, exploring the underlying chemical principles, resulting material properties, and the advantages, limitations, and potential applications of each method.2.1 Direct aqueous-phase synthesis
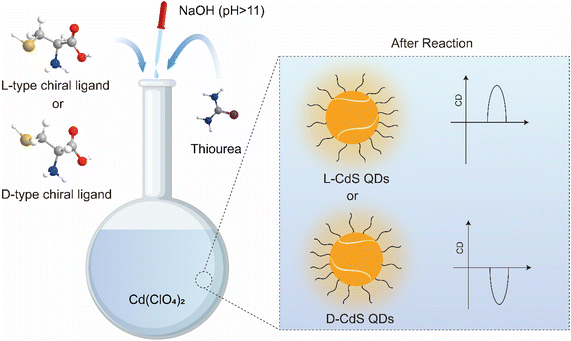
The direct aqueous-phase synthesis of chiral QDs involves the precise combination of chiral ligands, ion sources, and reducing agents under alkaline conditions, with or without external energy input, to produce chiral QDs (Fig. 3). For chiral CdS or CdSe QDs, the synthesis process begins with the introduction of a cation source, typically a Cd2+ precursor such as cadmium acetate or cadmium chloride, into the reaction mixture. The chiral ligand, exemplified by the thiol group (–SH) in Cys, coordinates with Cd2+ ions to form a Cd–Cys complex. Subsequently, an anion source, such as a selenium source (e.g. Na2SeO3) or a sulfur source (e.g. Na2S or thiourea), is added to generate Se2− or S2− anions, which react with Cd2+ ions to nucleate CdSe or CdS. Under appropriate thermal and reaction conditions, these nuclei undergo growth into QDs. The chiral Cys ligand, through its coordinating ability, regulates growth kinetics and terminates the process, ensuring that the particles remain nanoscale and preventing the formation of bulk materials. Additionally, the chiral Cys ligand passivates the QD surfaces, minimizing defects and enhancing the optical properties and stability of the QDs. By meticulously designing and controlling these reaction steps, CdSe or CdS QDs with tailored sizes and optical characteristics can be synthesized, effectively preventing their aggregation into macroscopic materials. This method, systematically refined by Professor Gun'ko's team, has become a standard protocol for synthesizing various chiral QDs, including those of CdS, CdSe, CdTe, and doped variants.67 Similarly, Professor Lorenzo Branzi's team successfully synthesized AgInS2 QDs via room temperature coprecipitation in water, using chiral Cys as a capping agent (Fig. 4).42 Likewise, Professor Gil Markovich's team employed chiral Pen to direct the formation of chiral nanocrystals from the achiral phase of mercury sulfide (β-HgS).68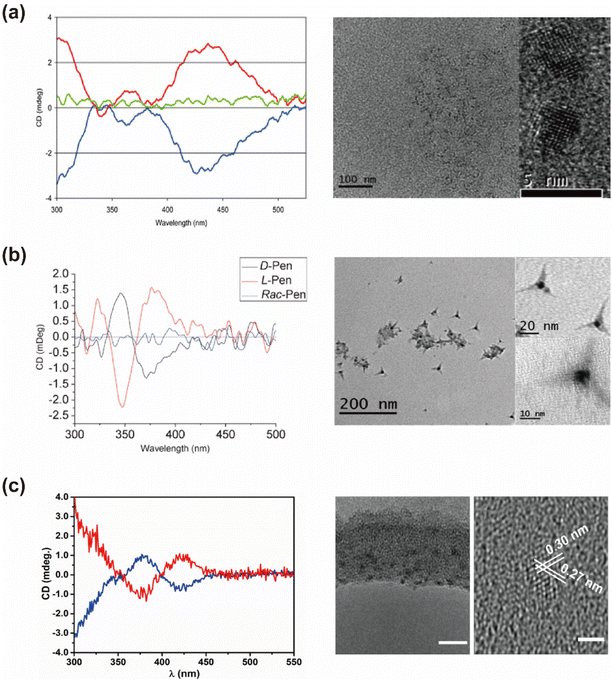 | ||
| Fig. 4 (a) CD spectra and TEM images of chiral Pen stabilized CdSe QDs. Reproduced with permission from ref. 39. Copyright 2010 Royal Society of Chemistry. (b) CD spectra and TEM images of chiral Pen stabilized CdS nano-tetrapods. Reproduced with permission from ref. 69. Copyright 2010 Royal Society of Chemistry. (c) CD spectra and TEM images of chiral AgInS2 QDs. Reproduced with permission from ref. 42. Copyright 2022 Royal Society of Chemistry. | ||
QDs synthesized through direct aqueous-phase methods typically exhibit pronounced chiral signals at the excitonic absorption peaks. For instance, CdS QDs synthesized with chiral Pen show a distinct circular dichroism (CD) signal at 400 nm,67 with the absorption dissymmetry factor (|gabs|) reaching 1.7 × 10−4. Similarly, CdS QDs prepared using chiral ferritin as the chiral source show significant chiral signals around 498 nm,69 accompanied by a luminescence dissymmetry factor (|glum|) of up to 4.4 × 10−3. Chiral Ag2S QDs exhibit strong chiral signals at around 719 nm,70 while chiral β-HgS QDs present notable chiral signals at around 207 nm,71 with a |gabs| value of 3.0 × 10−4. We systematically summarized the chiral and CPL properties of QDs synthesized via direct aqueous-phase methods (Table 1). The mechanisms underlying the generation of these chiral signals will be discussed in detail in Section 4.
| Types of QDs | |gabs| | |glum| | PLQY | Ref. |
|---|---|---|---|---|
| CdS@ferritin | N.A. | 4.4 × 10−3 | <20% | 69 |
| L-Pen CdS | 1.7 × 10−4 | N.A. | 12.8% | 67 |
| L-Cys CdTe | 0.3 × 10−4 | N.A. | N.A. | 72 |
| D-MeCys CdTe | 1.2 × 10−4 | N.A. | N.A. | 73 |
| L-Arg CdS | 0.3 × 10−4 | N.A. | N.A. | 74 |
| L-Pen CdSe | 0.4 × 10−4 | N.A. | 13.6% | 39 |
| L-Pen CdS tetrapods | 1.2 × 10−4 | N.A. | 24.5% | 75 |
| Ag2S QDs | N.A. | N.A. | 33% | 70 |
However, QDs synthesized via aqueous-phase methods typically exhibit lower absolute PLQY, often limited to around 20% or even lower, along with broader photoluminescence spectra.76,77 For instance, chiral CdS QDs demonstrate a full width at half maximum (FWHM) of 150 nm in their fluorescence spectra.61 These limitations stem from inherent challenges associated with aqueous-phase synthesis, including lower reaction temperatures, faster reaction kinetics, weaker interactions with chiral ligands, and the high polarity of the aqueous medium. These factors contribute to poor crystallinity and an increased number of defects within and on the surface of the QDs, which in turn elevate the likelihood of non-radiative recombination of electrons and holes, thereby reducing fluorescence brightness and broadening the overall fluorescence spectra.78–82
It is crucial to underscore that the quality of CPL is directly influenced not only by the material's asymmetry but also by the intrinsic fluorescence intensity of the QDs.48,61,68,74 The low PLQY presents a significant obstacle to achieving high-quality CPL in QD materials, restricting their research and application primarily to laboratory environments and spectral signal analysis. Consequently, the pursuit of both high luminescence/absorption dissymmetry and high PLQY remains a critical technical challenge for researchers striving to advance this field.
2.2 Post-synthetic modification
The post-synthetic modification approach, first introduced by Milan Balaz and colleagues in 2013, revolutionized the fabrication of chiral QDs.38 This method leverages high-temperature oil-phase synthesized QDs, which are renowned for their exceptional fluorescence brightness. Under strong alkaline conditions, chiral ligands with high binding affinities are introduced into the system. These ligands deprotonate in the basic environment, facilitating the displacement of the original oil-phase ligands—typically long-chain alkanes or other hydrophobic molecules—that detach more readily from the QD surface under such conditions (Fig. 5). This process enables the successful ligand exchange, effectively transferring the QDs from an oil-phase solvent to an aqueous system, thereby producing chiral QDs. This technique has been widely employed to functionalize a variety of chiral QDs, including CdS,83,84 CdSe,85,86 CdTe,87,88 CuInS2,43 CdSe@ZnS,89–91 and InP. It is also applicable for imparting chirality to semiconductor materials of various morphologies, such as tetrahedral, tetrapods, and quantum rods.47,92Through this process, distinct mirror-image chiral signals and CPL peaks can be observed at the excitonic absorption peaks of the QDs (Fig. 6). The establishment of this method has significantly enhanced the study of chirality induction and CPL properties in QDs. For instance, using this ligand exchange technique, chiral Cys ligands can be coated onto non-chiral CdSe QDs synthesized in the oil-phase to produce chiral CdSe QDs, which exhibit distinct CPL with a |glum| value of 4 × 10−3.38 Professor Gaoling Yang's team successfully synthesized L/D-Cys CdTe QDs using ligand-induced chirality, which displayed pronounced mirror-image chiral signal peaks at 248 nm, along with fluorescence enhancement upon interaction with amino acids.88 Similarly, Professor Tingchao He's team successfully synthesized CdSe/CdS nanocrystals with various morphologies, including nanoflowers, tadpoles with one to three tails, and point/rod structures. These nanocrystals displayed tunable absorption and luminescence properties, with CPL signal peaks at 585 nm and the maximum |glum| value reaching 8.4 × 10−4.49 Likewise, Professor Zhiyong Tang's team synthesized two different crystal types of chiral CdSe nanoplatelets using this method. These nanoplatelets exhibited opposite CD signal peaks at the first excitonic transitions in wurtzite and zinc blende nanoplatelets, both capped with the same chiral Cys ligand.93 QDs obtained through this method typically exhibit refined CD spectra, which are closely linked to the hybridization between the ligand highest occupied molecular orbital (HOMO) and the QD valence band states.88,94 The CD spectra demonstrate high sensitivity to the structural details and interactions between the ligands and the QDs.83 This aspect will be further elaborated in the spectral regulation section.
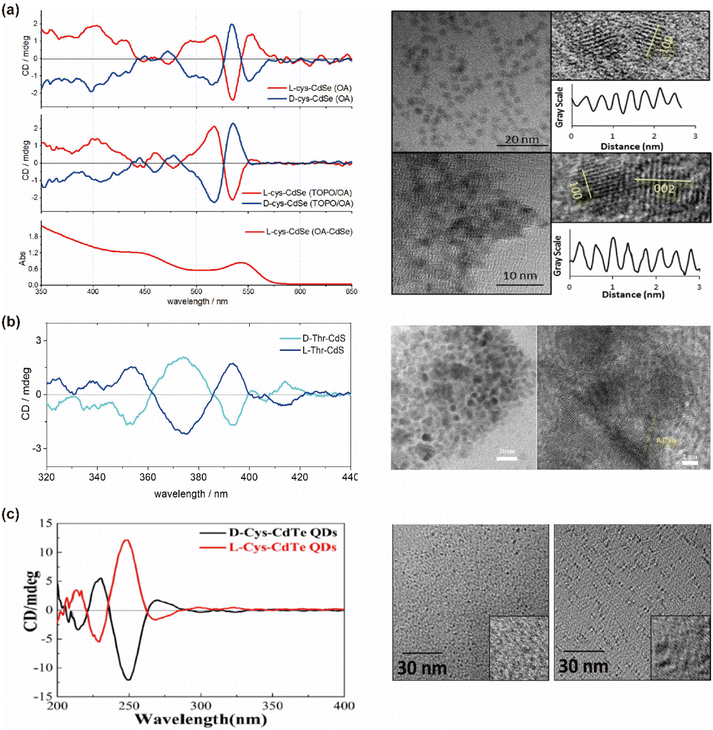 | ||
| Fig. 6 (a) CD spectra and TEM images of oleic acid capped CdSe and L-Cys capped CdSe QDs by post-synthetic modifications. Reproduced with permission from ref. 85. Copyright 2013 American Chemical Society. (b) CD spectra and TEM images of chiral CdS QDs. Reproduced with permission from ref. 84. Copyright 2023 Elsevier. (c) CD spectra and TEM images of chiral CdTe QDs. Reproduced with permission from ref. 88. Copyright 2020 American Chemical Society. | ||
However, the post-synthetic modification process can inadvertently lead to a significant reduction in fluorescence brightness and decreased material stability.95 Although native ligands are typically replaced without substantially altering the composition and structure of the nanocrystal core, the ligand exchange during post-synthetic modification often disrupts the surface structure of the QDs. This disruption can cause lattice mismatch, activating additional mid-gap states that facilitate non-radiative recombination, resulting in a sudden decline in CPL activity.80 The introduction of numerous surface defect states lowers the PLQY to below 20% and broadens the FWHM of the emission spectra. To overcome these challenges, researchers have turned to Type I core–shell QDs as starting materials for post-synthetic modification. The bandgap distribution in core–shell structures effectively confines electrons and holes within the core. Encapsulating the luminescent core with a wider bandgap shell ensures that the emission properties are either unaffected or minimally affected by external conditions, thereby improving the PLQY and enhancing environmental stability. While this strategy efficiently maintains the PLQY above 40%, the shielding effect of the inorganic shell tends to weaken the intensity of the chiral signals.96 The impact of the shell on the chiral and CPL signals of QDs will be discussed in detail in Section 3.3. Consequently, achieving high brightness and strong chiral/luminescence dissymmetry in CPL QDs remains a formidable challenge. A summary of representative studies employing this method is provided in Table 2.
| Types of QDs | |gabs| | |glum| | PLQY | Ref. |
|---|---|---|---|---|
| L-Pen CdSe QDs | 8.0 × 10−4 | 3.0 × 10−3 | N.A. | 85 |
| L-Cys CdSe@CdS QRs | 2.0 × 10−4 | 4.6 × 10−5 | 54% | 47 |
| L-Cys CdS QDs | 0.8 × 10−4 | N.A. | N.A. | 48 |
| L-Cys CdSe@CdS QDs | 4.0 × 10−5 | N.A. | 19% | 62 |
| Cys CdSe/CdS QDs | 3.0 × 10−4 | N.A. | 4% | 96 |
| L-Thr CdS QDs | 6.8 × 10−4 | N.A. | N.A. | 84 |
| L-Cys CdTe/CdSe QDs | 2.4 × 10−5 | N.A. | N.A. | 97 |
| D-Cys CdSe/CdS QRs | 9.4 × 10−5 | 8.4 × 10−4 | 44% | 49 |
| D-Cys CdSe/CdS Tadpole | 9.8 × 10−5 | 5.1 × 10−4 | 37% | 49 |
2.3 Water/oil interface mediated synthesis
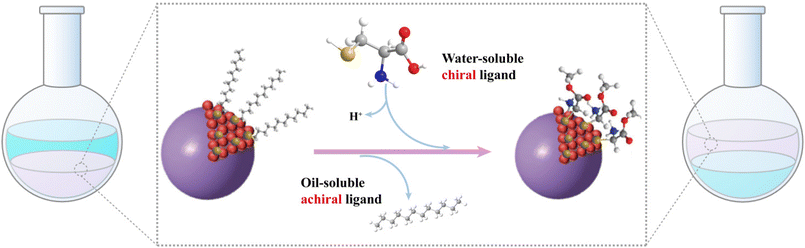
In recent advancements, our team has pioneered an innovative approach for synthesizing CPL CdSe@ZnS QDs at the water/oil interface, achieving a synergistic enhancement of both fluorescence brightness and emission dissymmetry.98 This process begins with oleic acid (OA)-capped CdSe QDs, synthesized in the oil phase and dissolved in chloroform, serving as the seed material. An alkaline aqueous solution containing chiral histidine (His), ZnCl2, and thiourea (SC(NH2)2) is used as the shell growth solution. The immiscible biphasic system is thoroughly mixed to initiate a reaction at the water/oil interface. During this process, the chiral ligands preferentially coordinate with Zn2+ ions, forming complexes that compete with the original long-chain alkanes on the QD surface. These complexes are drawn to the CdSe surface through carboxyl interactions and subsequently combine with slowly released S2− ions in the solution, forming a chiral ZnS shell (Fig. 7). Following the interface reaction, the QDs transition from the lower oil phase to the upper aqueous phase, achieving a yield exceeding 98%. The resultant QDs exhibit a remarkable improvement in absolute PLQY, increasing from 5.2% in the CdSe seed material to 67.2%. Additionally, both the absorption and emission dissymmetry g-factors reach 0.01 (Fig. 8). These values significantly surpassed the previously reported PLQY of less than 40% and chiral asymmetry factors ranging from 10−5 to 10−2 for CPL/chiral QDs. This innovative approach marks a significant advancement in the field of CPL QDs.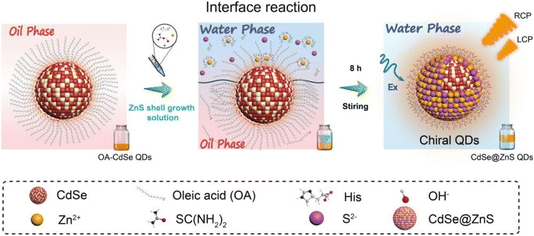 | ||
| Fig. 7 Schematic diagram of high brightness CPL QDs synthesized based on water/oil interface. Reproduced with permission from ref. 98. Copyright 2023 American Chemical Society. | ||
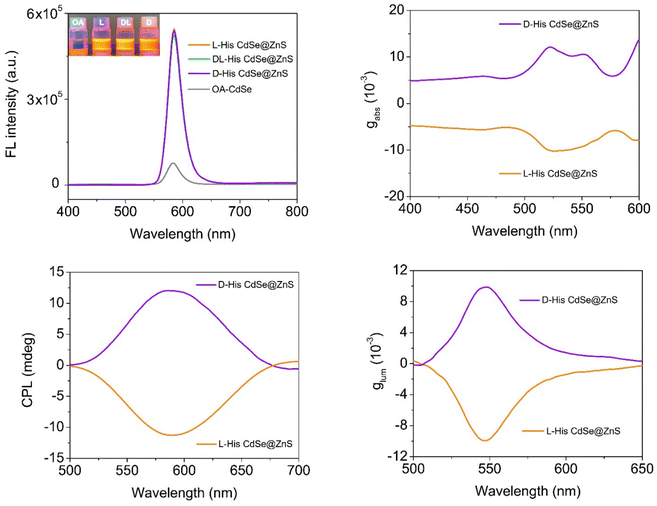 | ||
| Fig. 8 Optical signal characterization of CPL QDs synthesized at the water/oil interface. Reproduced with permission from ref. 98. Copyright 2023 American Chemical Society. | ||
The distinctive advantage of the water/oil interface reaction method lies in its ability to combine the high PLQY characteristic of post-synthetic modification with robust chiral signals achieved through direct aqueous synthesis. This technique utilizes the CdSe core for enhanced brightness and the ZnS shell for chiral asymmetry. The ZnS shell, with an approximate thickness of 2.6 atomic layers, facilitates orbital hybridization between the chiral ligands and the luminescent core of the CdSe QDs, resulting in outstanding optical performance. Consequently, this method successfully produces QDs with both high PLQY and pronounced chiral emission dissymmetry.
We compared the distinct characteristics of the three aforementioned synthesis methods, as summarized in Table 3. The aqueous-phase synthesis method is noted for its rapid reaction rates and strong chiral luminescence properties, making it easy to prepare materials with high production yields. However, its relatively low PLQY restricts its use in various optical imaging systems. Conversely, post-synthetic modification produces chiral/CPL QDs with high PLQY and finely tuned chiral spectra that are highly responsive to interfacial changes. Despite these advantages, this method faces challenges such as low preparation yields and a tendency toward fluorescence quenching. Moreover, enhancing chiral signals remains a significant obstacle for further development and practical applications. The water/oil interface reaction method demonstrates considerable promise in enhancing both CPL and fluorescence brightness of QDs. However, this approach has yet to achieve widespread adoption, and its general applicability across different materials still requires further validation.
| Specific feature of QDs | Advantages | Limitations | |
|---|---|---|---|
| Direct aqueous-phase synthesis | • QDs can be produced in aqueous phase | • High CD anisotropy | • Requires precise control of time and temperature |
| • Low quantum yield | • Ease of synthesis | • Limited control over shape and size of QDs | |
| • Defect luminescence with a very broad PL band | • The absence of toxic long chain organic molecules | • Yields are quite low | |
| • Large size distribution | • Excellent aqueous stability | ||
| • Suitable for biological applications | |||
| Post-synthetic modification | • Transfer of QDs from the oil phase to the aqueous phase | • Refined CD spectra | • Cumbersome synthesis steps |
| • Narrow size distribution | • Relatively easily functionalized with various chiral ligands | • Oil phase synthesis requires higher temperatures | |
| • Good quantum yield | • Easy to control the size and shape of QDs | • QDs partially lose quantum efficiency and become less stable after phase transfer | |
| • Clear exciton absorption bands | • Facilitates modulation of CD and CPL signals | • Excessive shell thickness inhibits chiral signal | |
| • Few crystal defects | |||
| Water/oil interface mediated synthesis | • Transfer of QDs from the oil phase to the aqueous phase | • Low phase transfer losses | • Currently less applicable |
| • Higher quantum yield | • Simultaneous increase in PLQY and asymmetry g-factor | • Oil phase synthesis requires higher temperatures | |
| • Thin chiral shell layer | • Simplified operational steps | ||
| • Higher asymmetry g-factor; | • Good stability | ||
| • Fewer crystal defects |
3 Fine-tuning chiroptical activity
Beyond the synthesis methods, fine-tuning chiral ligands, QD size, and QD structure plays a pivotal role in shaping the chiral and CPL properties of QDs. Such regulation is essential for synthesizing high-quality chiral/CPL QDs and deepening our understanding of the origins of chirality and CPL signals in these materials. In this section, we explored in detail how chiral ligands, QD size, and QD structure influence and regulate spectral signals. This comprehensive analysis aims to uncover the fundamental principles governing spectral changes, providing valuable insights for both the synthesis and mechanistic studies of high-quality chiral QDs.3.1 Chiral ligand-regulated chiroptical activity of QDs
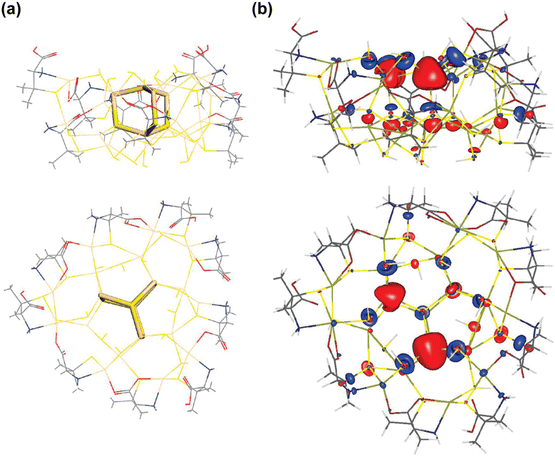
The chiroptical activity of QDs, encompassing the position and intensity of chiral signals as well as signal inversion, is influenced by an interplay of factors including the ligand type,46 surface ligand conformation,99 bonding mode of ligands with QDs,100 and the number of stereocenters in the ligands.94 We reviewed representative studies to illustrate how chiral ligands influence the chiroptical properties and CPL of QDs.Chiral small molecules, such as chiral amino acids, are commonly used in the synthesis of chiral QDs.101 Thiol-containing chiral molecules are particularly favored due to their strong covalent bonding with metal atoms.102,103 For instance, research by Professor Milan Balaz demonstrated that L- and D-Cys chiral thiol capping ligands could induce chiroptical properties in achiral CdSe QDs.85 Cys-capped CdSe QDs, derived from achiral OA-capped CdSe QDs through post-synthetic ligand exchange, exhibited size-dependent electronic CD and CPL. Notably, opposite CPL signals were observed for CdSe QDs capped with D- and L-Cys. Magic angle spinning solid-state NMR (MAS ssNMR) experiments suggested a bidentate interaction between Cys and the CdSe surface (Fig. 9). Therefore, thiol-containing small molecules impart chiroptical activity to QDs through the synergistic action of carboxyl and thiol groups, affecting both CD and CPL properties. Similarly, thiol-containing ligands, such as Cys derivatives, GSH, and Pen, are used in post-synthetic modification methods to endow QDs with chirality.
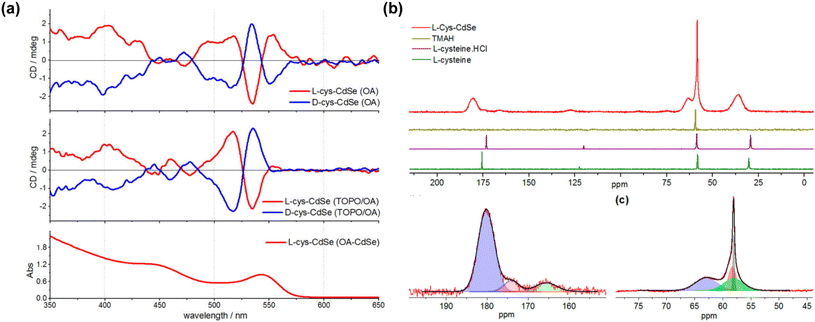 | ||
| Fig. 9 (a) CD and UV-vis absorption spectra of Cys-capped CdSe QDs. (b) MAS ssNMR of L-Cys CdSe QDs. Reproduced with permission from ref. 85. Copyright 2013 American Chemical Society. | ||
Beyond thiol-containing ligands, carboxyl groups in ligands also play a crucial role in imparting chiroptical activity to QDs.46,94,99,104 There have been reports of inducing chirality in CdSe QDs using thiol-free chiral carboxylic acid capping ligands, such as L- and D-malic and tartaric acids.46 CD and infrared spectroscopy data indicate that the presence of chiral carboxylic acid capping ligands on the CdSe QD surface is necessary but not sufficient for inducing optical activity in QDs. Successful induction of chirality by chiral dicarboxylic acid capping ligands during phase transfer ligand exchange requires three oxygen donor groups. These three-donor group ligands preferentially bind in one of two possible orientations on the QD (111) surface, producing a CD signal, while bidentate binding results in near equal energies for the binding orientations, leading to cancellation of the CD signals. Furthermore, Professor Vivian E. Ferry's team demonstrated that directly adding chiral ligands to CdSe QDs stabilized in a nonpolar solution by oleic and myristic acids results in significant chiral signal enhancement.94 Stirring the CdSe QDs with chiral carboxylic acids and carboxylates in toluene induces ligand exchange and precipitation, revealing that dicarboxylic acid systems are more effective than monocarboxylic acids in amplifying chiral signals. The more detailed study by Professor Milan Balaz's team revealed that an increased number of stereogenic centers do not directly translate to higher CD anisotropy.105 Rather, the configurational “match/mismatch” originating from multidentate binding of the ligand and ligand–ligand interactions on the surface of QDs must be taken into consideration. The research of Professors Yong Yan and Tianyong Zhang focused on the electron-withdrawing capacity of the substituents on the asymmetric carbon of carboxylic acid ligands.99 They found that the electron-withdrawing nature of these substituents significantly influences the chiroptical activity of QDs, with stronger electron-withdrawing groups inducing more intense chiral signals. Notably, in monocarboxylic acid systems with strong electron-withdrawing groups, the resulting chiroptical activity of the QDs can surpass that of dicarboxylic acid systems (Fig. 10).
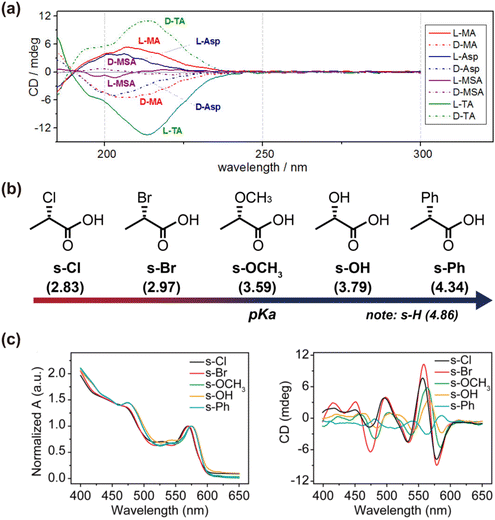 | ||
| Fig. 10 (a) Structural effects of thiol-free carboxylic acid ligands on chiral CdSe QDs. Reproduced with permission from ref. 46. Copyright 2017 American Chemical Society. (b) Chemical structures of five chiral monocarboxylic acid ligands is changed on the asymmetric carbon atom substituents. (c) UV-vis absorption and CD spectra of chiral CdSe QDs induced by using chiral monocarboxylic acids. Reproduced with permission from ref. 99. Copyright 2021 IOP Publishing. | ||
In summary, comparisons of QDs functionalized with different carboxylic and thiol-containing chiral ligands reveal that both types of ligands can effectively induce chiroptical activity, with neither showing definitive superiority (Table 4). The strength of the induced chiral signals largely depends on the binding affinity between the ligands and the QD surface, emphasizing the importance of selecting ligands based on specific material interactions.
| Types of QDs | Ligands | Solvent | |gabs| | λ CD | Ref. |
|---|---|---|---|---|---|
| CdSe QDs | L-Cys | H2O | 1.9 × 10−4 | 569 nm | 85 |
| CdS QDs | L-Cys | H2O | 0.8 × 10−4 | 530 nm | 48 |
| CdSe@CdS QDs | L-Cys | H2O | 4.0 × 10−5 | 560 nm | 62 |
| CdSe QDs | N-Acetyl-L-cysteine | H2O | 0.5 × 10−4 | 584 nm | 40 |
| CdS QDs | L-Thr | MeOH | 6.8 × 10−4 | 387 nm | 84 |
| CdTe/CdSe QDs | L-Cys | H2O | 2.4 × 10−5 | 647 nm | 97 |
| CdSe QDs | L-MA | Na-cacodylate buffer | 4.4 × 10−5 | 539 nm | 46 |
| CdSe QDs | L-TA | Na-cacodylate buffer | 5.6 × 10−5 | 515 nm | 46 |
| CdSe QDs | N-Acetyl-L-aspartic acid | DMF | 6.0 × 10−5 | 528 nm | 94 |
| CdSe QDs | L-Tartaric acid | DMF | 7.0 × 10−5 | 528 nm | 94 |
| CdSe QDs | L-Malic acid | DMF | 3.6 × 10−5 | 528 nm | 94 |
| CdSe QDs | 2-Chloropropionic acid | CHCl3 | 4.8 × 10−4 | 570 nm | 99 |
| CdSe QDs | 2-Bromopropionic acid | CHCl3 | 5.8 × 10−4 | 570 nm | 99 |
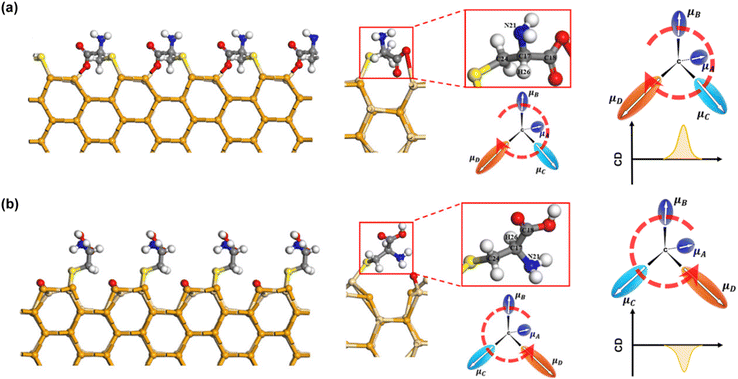
In addition to the type and absolute configuration of chiral ligands, their conformation plays a crucial role in regulating chiroptical activity. For example, when CdSe QDs are functionalized with N-acetyl-L-cysteine and L-homocysteine, they exhibit “mirror-image” CD spectra.40 This illustrates that altering a ligand's structure, rather than reversing its absolute configuration, can invert the CD signals of the QDs. The chiral inversion results from the different binding modes of N-acetyl-L-cysteine and L-homocysteine on the CdSe QD surface (Fig. 11). This suggests that the induced chirality in QDs is not merely due to simple orbital mixing between the electronic states of chiral ligands and achiral QDs. Instead, the induced chirality heavily depends on the binding geometry and conformation of the ligands. This deepens our understanding of ligand-induced chirality in QDs and highlights the influence of the QDs themselves on the conformation of anchoring ligands. The study also revealed that adding L- and D-Cys to solutions of L-/D-Cys-functionalized CdTe QDs creates hetero- or homochiral complexes.88 Homochiral amino acids enhance the CD signal, while heterochiral ones weaken it, likely due to different binding configurations of Cys molecules on the QD surface.
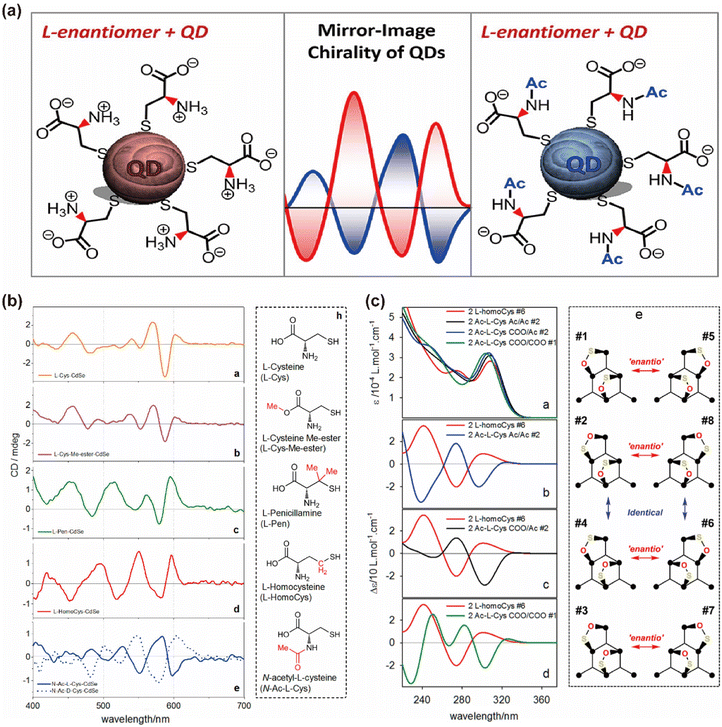 | ||
| Fig. 11 (a) CdSe QDs chiral inversion via structural changes in thiol-containing chiral ligands. (b) CD spectra of CdSe QDs functionalized with the corresponding ligands. (c) Simulated UV and CD spectra of selected model CdSe complexes with two L-homocysteine and N-acetyl-L-cysteine ligands. Reproduced with permission from ref. 40. Copyright 2016 American Chemical Society. | ||
Notably, beyond the influence of chiral ligands, the impact of non-chiral ligands tightly bound to the QD surface should not be overlooked. Studies have shown that QDs synthesized with different native ligands, such as OA and trioctylphosphine oxide (TOPO), and subsequently functionalized with Cys can exhibit surprising chiral signal inversion (Fig. 12).95 This inversion can be attributed to two main factors: the differences in the lattice structures of QDs synthesized with different ligands, and the varying effects of non-chiral ligands on the binding of chiral ligands. Thus, the ligand-induced synthesis process can be analogously compared to a single-molecule nucleophilic substitution reaction (SN1), where both reaction equilibrium and kinetics depend not only on the nature of the nanocrystalline nuclei and chiral molecules but also on the presence of natural non-chiral ligands. Different non-chiral ligands with varying polarities can influence the surface coverage and molecular arrangement of chiral Cys on the QD surface, thereby affecting the chiral signal.
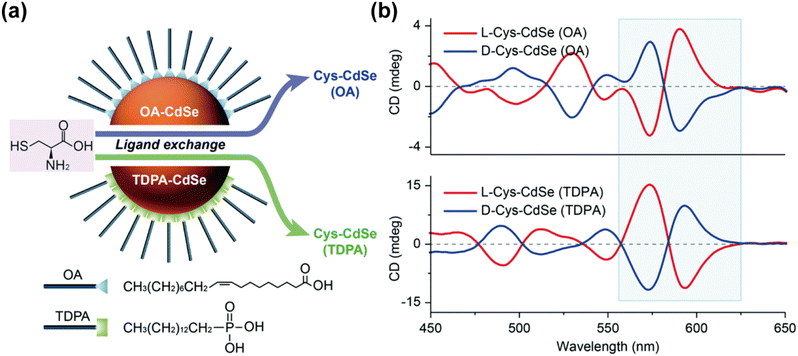 | ||
| Fig. 12 (a) Synthesis of chiral CdSe QDs by post-synthetic modification. (b) Effects of natural achiral ligands on chiral CdSe QDs. Reproduced with permission from ref. 95. Copyright 2021 Royal Society of Chemistry. | ||
3.2 Effect of QD size on chiroptical activity
The size of QDs is a critical factor influencing their band gap, which significantly affects their chiroptical activity.38,83,85,106 In a seminal study by Professor Gil Markovich's team, chiral Pen-capped CdS and CdSe QDs of various sizes were synthesized, revealing a notable decrease in chiroptical activity with an increase in QD size.106 They established a correlation curve between the inverse of QD size and chiroptical activity, attributing the signal attenuation to the interplay of surface effects and electron–hole pair interactions. In smaller QDs, the higher proportion of surface atoms amplifies the influence of surface ligands, resulting in more pronounced chiral signals. Conversely, as QD size increases, the proportion of surface atoms diminishes, leading to weaker surface effects and reduced chiroptical activity. Furthermore, the electronic structure and band gap of QDs vary with size. Smaller QDs, due to quantum confinement effects, exhibit larger energy level separations, enhancing electron–hole pair interactions and potentially strengthening chiral signals. In contrast, larger QDs display reduced quantum confinement effects, smaller energy level separations, and weaker electron–hole pair interactions, which may attenuate chiral optical signals. In other systems, an increase in QD size has been associated with enhanced chiroptical activity, reaching a maximum at specific dimensions. For instance, in chiral CdSe QDs synthesized through chiral Cys ligand exchange, both the CD profile and CD anisotropy were found to vary with the size of the CdSe nanocrystals.85 Increasing the diameter led to a red shift in the cotton effect. Progressive spectral changes were observed with an increase in the size of L-Cys CdSe QDs, with new CD bands emerging at shorter wavelengths for diameters greater than 4.1 nm. The most pronounced cotton effect at the band gap wavelength was observed in L-Cys CdSe with a diameter of 4.4 nm. Although the CD anisotropy of Cys-CdSe QDs varied with size, the changes in the absorption dissymmetry factor gabs were minimal, indicating no significant correlation between the diameter of CdSe QDs and its g value (Fig. 13). In summary, the relationship between QD size and chiroptical activity is governed by a complex interplay of factors, including band gap variations, surface ligand interactions, and surface area-to-volume ratios. A comprehensive analysis considering these interactions is essential to fully understand the impact of QD size on chiroptical properties, rather than focusing on isolated factors.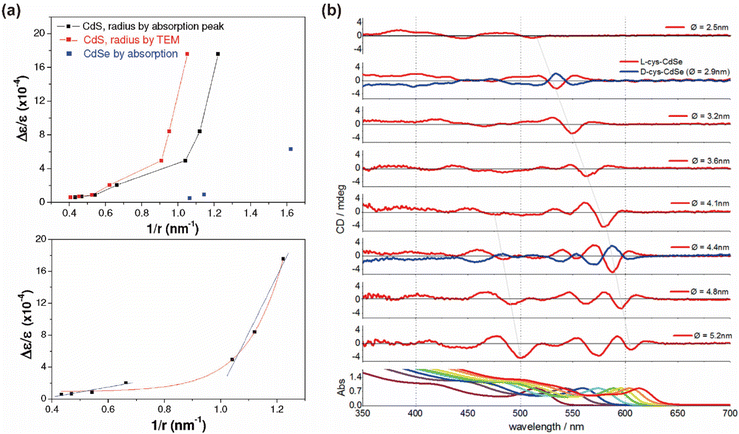 | ||
| Fig. 13 (a) Size dependence of the chiroptical activity in QDs. Reproduced with permission from ref. 106. Copyright 2011 American Chemical Society. (b) Size-dependence CD spectra and UV-vis absorption of chiral CdSe QDs. Reproduced with permission from ref. 85. Copyright 2013 American Chemical Society. | ||
3.3 Core–shell structures in chiral QDs
In the study of chiral QDs, core–shell structures have garnered extensive attention.97,107 This is primarily due to the propensity of single-component QDs to interact with ligands and the external environment during post-synthesis modification, leading to the formation of numerous surface trap states. These trap states can significantly reduce the fluorescence brightness of QDs, or even cause fluorescence quenching, complicating the production of high-quality circularly polarized or chiral QDs. Core–shell QDs, particularly type-I core–shell QDs, utilize the wider bandgap of the shell to encapsulate the luminescent core, thereby confining electrons within the core and shielding it from environmental influences, which ensures a high PLQY.108,109 The post-synthetic modification of core–shell QDs with chiral ligands to impart chiroptical activity has been demonstrated to be feasible. Professor Ute Resch-Genger's team functionalized CdSe@CdS QDs with chiral Cys ligands, analyzing the relationship between exciton energy levels, PLQY, and CD spectra for QDs with varying shell thicknesses (Fig. 14).96 Similar studies have focused extensively on the influence of shell thickness on the chiroptical activity of QDs.97,107,110,111 These results indicate that the presence and thickness of the shell layer affect the electronic structure of QDs, thereby influencing their chiroptical activity. It was observed that as the shell thickness of core–shell QDs increased, there was a significant enhancement in PLQY due to the effective passivation of surface defects by the inorganic shell. However, for chiroptical activity, an increase in shell thickness is typically associated with a notable decrease (Fig. 15). This reduction can be attributed to the increased distance between the ligands and the local holes in the CdSe core, leading to reduced coupling with the chiral source. In some studies, an initial enhancement in CD signals with an increase in shell thickness, followed by a reduction, has been observed.62,97 This phenomenon is often seen in systems with finely tuned shell layers. For instance, CdSe@CdS QDs with 1-3 CdS atomic layers exhibit enhanced CD signals, which then decrease as the shell thickness increases to 4 and 5 CdS atomic layers. The initial enhancement is likely due to exciton leakage through the thinner shell, which does not hinder the orbital hybridization between the QDs and the chiral ligands. The subsequent reduction in CD intensity with thicker shells can be explained by the shell acting as an energy barrier for holes, thus diminishing CD strength.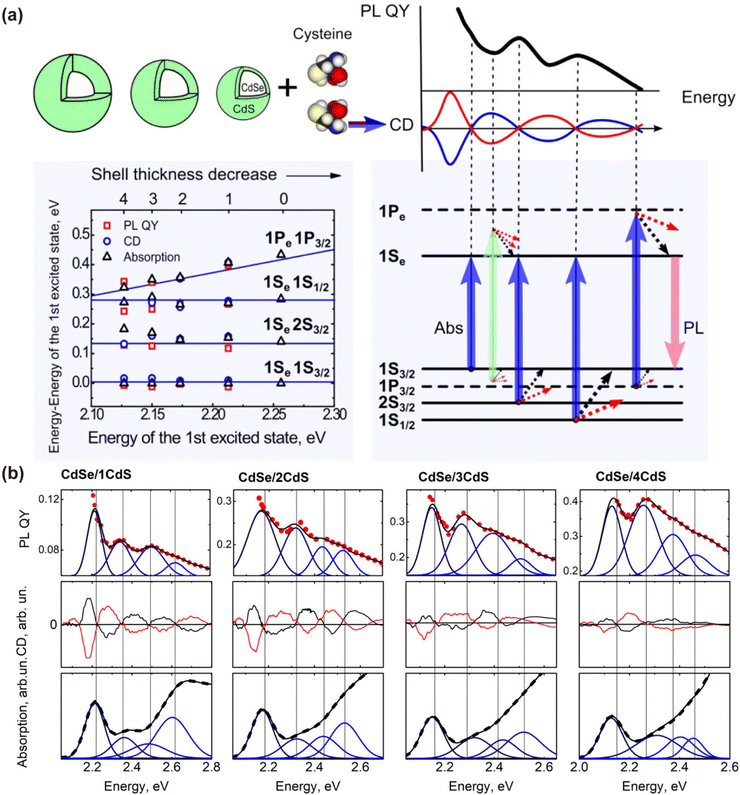 | ||
| Fig. 14 (a) The first electronic energy transitions and the first four lowest energy transitions in CdSe/nCdS QDs. (b) Analysis of the EED of PLQY, CD spectra, and absorption spectra of CdSe/nCdS QDs. Reproduced with permission from ref. 96. Copyright 2017 American Chemical Society. | ||
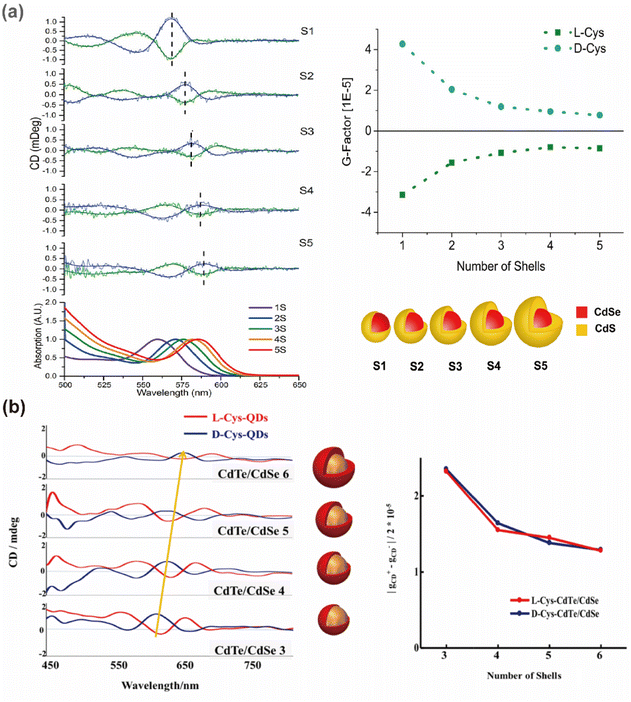 | ||
| Fig. 15 Effect of the shell layer thickness on the absorption and CD spectra of chiral (a) CdSe@CdS core–shell QDs, reproduced with permission from ref. 62, copyright 2017 American Chemical Society, and (b) CdTe/CdSe core–shell QDs. Reproduced with permission from ref. 97. Copyright 2021 Wiley. | ||
Beyond type-I core–shell QDs, the impact of inverse band alignment on chiroptical activity has also been explored. For example, comparing the chiroptical differences between CdSe@CdS and CdS@CdSe QDs (Fig. 16).83 In these systems, the holes are located in different regions (the core in CdSe@CdS and the shell in CdS@CdSe), providing a platform to verify the dominant role of hole-ligand coupling in chiral induction. Monitoring the variation in CD amplitude with an increase in shell thickness in both core–shell configurations, it was found that when electrons are confined within the shell, the chiroptical activity is significantly shielded as the shell thickness increases. Conversely, in the opposite core/shell system, where the larger bandgap material (CdS) is in the core and the CdSe with the higher valence band is in the shell, it is expected that the hole-surface interaction remains unimpeded, and CD does not diminish with an increase in shell thickness. However, the luminescence performance remains highly susceptible to environmental influences, further confirming that CD induction is highly sensitive to the specific adsorption conformation and chemical bonding properties of ligands on the surface.
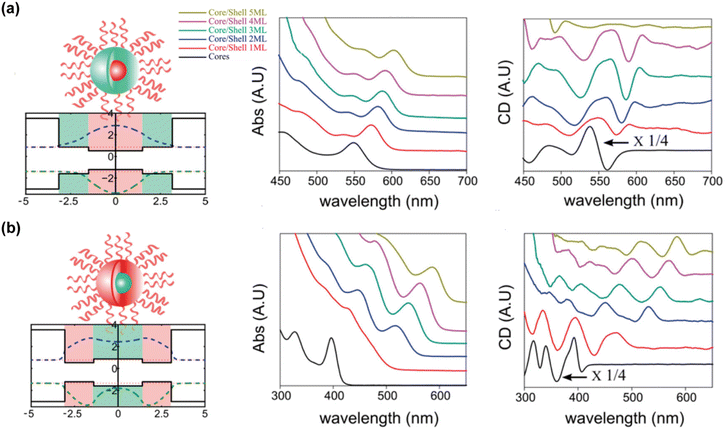 | ||
| Fig. 16 Absorption and CD spectra of (a) CdSe/CdS and (b) CdS/CdSe core/shell QDs. Reproduced with permission from ref. 83. Copyright 2016 American Chemical Society. | ||
In conclusion, the intricate interplay between chiral ligand interactions, QD size, shell thickness, and electronic structure underscores the complexity of core–shell QDs and their potential for advanced chiroptical applications. Understanding these multifaceted interactions is crucial for the development of high-performance chiral QDs.
4 Theoretical calculations for CPL generation in QDs
CPL signals in QDs are intrinsically linked to the fluorescence and chiral properties of the material, serving as a direct representation of the polarization information of the emitted light. Developing high-brightness CPL QDs necessitates a thorough understanding of the generation and modulation mechanisms of CPL, which can guide the optimization of chirality, fluorescence, and CPL signals to achieve superior material performance. Thus, we reviewed theoretical calculations that aim to elucidate the CPL characteristics of QDs, providing theoretical guidance and technical support for the next steps in developing high-performance CPL QDs.4.1 Theoretical basis of CPL
The CPL properties of a material reflect the electronic transition information of its excited states, commonly observed in chiral fluorescent materials.1–3,112,113 CPL properties are primarily quantified by the glum value and the brightness of the circularly polarized emission. The emission dissymmetry factor is derived from the difference in intensity between left- and right-circularly polarized spontaneous emissions (ΔI) from inherently chiral fluorescent substances or those in a chiral environment, as measured by CPL spectroscopy:112,114,115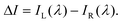 | (1) |
Given the difficulty in precisely obtaining absolute I and ΔI values, the degree of asymmetry in CPL is quantified by the relative intensity difference of left- and right-circularly polarized emissions, known as glum:116,117
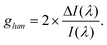 | (2) |
As indicated in the equation, glum values range between −2 and 2. Currently, some self-assembled supramolecular materials exhibit glum values approaching these extremes (±2).7 However, research on inorganic QD materials is still in its infancy, with glum values typically ranging from 10−5 to 10−2.118
Enhancing the glum values of materials is a primary research focus. According to Rosenfeld's theory, the glum equation can be re-expressed in terms of the electric dipole (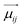 ) and magnetic dipole (
) and magnetic dipole (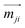 ) parameters of electrons:2,117,119–121
) parameters of electrons:2,117,119–121
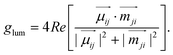 | (3) |
For non-magnetic materials, where the electric dipole typically dominates over the magnetic dipole, this equation is simplified as:
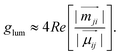 | (4) |
Thus, the CPL properties of materials can be modulated by increasing the magnetic dipole strength or decreasing the electric dipole strength.22,114,122
In addition to emission dissymmetry, another crucial property of materials is CPL brightness. CPL brightness can be evaluated through parameters such as the figure of merit (FM), asymmetry quantum yield (φa), and CPL brightness (BCPL):120,123
| FM = Φ × glum | (5) |
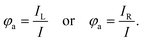 | (6) |
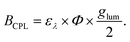 | (7) |
The chirality of QDs is closely related to CPL properties, reflecting asymmetries in the ground and excited states of the material, respectively. Measurement of the chiral signal usually relies on CD spectroscopy, based on the difference in absorption of left and right CPL by the material, which is commonly described by the absorption asymmetry factor gabs:124,125
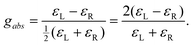 | (8) |
In this case, CD arises from chiral electronic displacements along helical paths involving simultaneous translations and rotations of charges, corresponding to non-orthogonal, non-zero electric and magnetic dipole moments of jumps, with both electric and magnetic dipole moments playing an important role. Thus, gabs can be further described as:
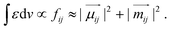 | (9) |
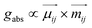 | (10) |
From the above equations, it can be seen that the chiral signal of a substance is positively correlated with the electric and magnetic dipole moments in terms of absorption asymmetry. However, CPL and chirality show some inconsistency in the correlation of the electronic dipole moment. Specifically, glum is proportional to the magnetic dipole of the electron and inversely proportional to the electric dipole.
4.2 Mechanisms of CPL in QDs
Understanding the mechanisms behind CPL in QDs is crucial for their effective manipulation and application. Extensive research has been conducted to elucidate the chiral mechanisms in QDs, aiming to model and reproduce spectra to explain the origins of chiroptical activity.Professor Milan Balaz's team employed Time-Dependent Density Functional Theory (TDDFT) to verify that attaching L- and D-Cys to the surface of QDs induces measurable opposite CD signals for the excitonic band of the QDs.85 The molecular orbitals primarily involved in excitonic band transitions are delocalized over both the inorganic QDs and the ligand, including the HOMO. In contrast, the virtual orbitals (LUMO, LUMO+1, and LUMO+2) remain localized on the QDs. This hybridization of the CdSe highest occupied orbitals with the orbitals of the chiral ligand appears to be the primary source of induced chirality in QDs (Fig. 17). This theoretical approach has been widely accepted and applied to understand the CD spectra of inorganic QDs with various amino acids,40,48 QD sizes,83 shell thicknesses,62 and the properties and binding modes of ligands.100
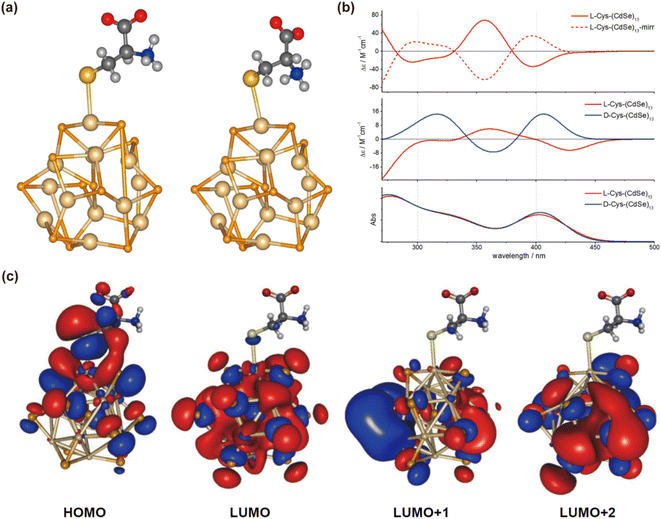 | ||
| Fig. 17 (a) Optimized geometries of L-Cys and D-Cys CdSe QDs. (b) Theoretical simulations of CD and UV-vis spectra of model Cys-CdSe QDs. (c) Calculated molecular orbitals of L-Cys CdSe QDs. Reproduced with permission from ref. 85. Copyright 2013 American Chemical Society. | ||
In 2011, Professor Zhiyong Tang's team attempted to use the Discrete Dipole Approximation (DDA) to simulate and understand the relationship between CD spectra and QD size (Fig. 18).126 In this model, the chiral molecule, with polarizabilities α1L and α1R (for left- and right-polarized light, respectively), is coupled with an achiral nanocrystal (NC) with polarizability α2. The interaction between the chiral molecule and the NC generates dipoles p1 and p2. The effective polarizability of the NC is a mixture of the bare polarizabilities (α2, α1L/R) of both the NC and the chiral molecule. Since the polarizability of the chiral molecule depends on the light's polarization, the effective polarizability of the NC also becomes polarization-dependent due to the coupling with the chiral molecule, resulting in the CD signal observed experimentally. Additionally, Professor Tang's team developed a Nondegenerate Coupled-Oscillator Model (NDCO), which simplifies a CdSe quantum rod into three distinct chromophores.48 The CD peaks arise from the coupling of electric dipole transition moments among these chromophores, and the sign of the CD peaks is determined by the relative positions of the coupled chromophores. This model elucidates the sources of chiral signals and the crucial influence of electric dipole interactions on chirality in CdSe QDs (Fig. 19).
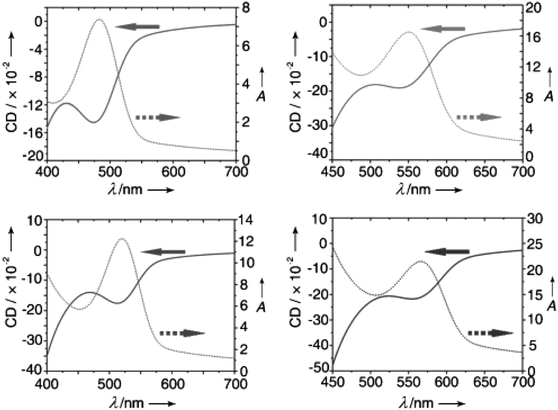 | ||
| Fig. 18 Size-dependent CD and UV-Vis spectra of D-GSH stabilized CdTe QDs of different sizes. Reproduced with permission from ref. 126. Copyright 2011 Wiley-VCH. | ||
 | ||
| Fig. 19 Schematic representation of the coupling between two electric dipole transitions in L-Cys stabilized CdSe quantum rods and the corresponding CD spectral analysis. Reproduced with permission from ref. 48. Copyright 2017 American Chemical Society. | ||
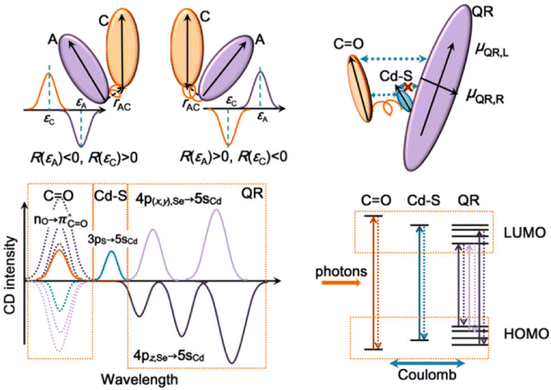
In addition to these methods, Density Functional Theory (DFT) and ab initio Molecular Dynamics (AIMD) have also been employed to analyze the stability of chiral amino acids on QDs, providing deeper insights into the origins of their chiroptical activity.88 For instance, DFT calculations have shown that Pen-mediated synthesis of chiral QDs results in significant chiral distortion on the QD surface, while deeper layers exhibit minimal distortion (Fig. 20).61 Based on DFT data, it has been proposed that surface defects within the chiral distortion layer of the QDs are responsible for the long-wavelength CD response. However, it is also possible that each deposited layer is chiral-distorted by the ligand during QDs synthesis in the chiral medium. This would not only lead to surface chiral defects but also structural chiral defects, contributing to the formation of chiral memory. Recently, Professor Xiaoqing Gao's team reported the chiral inversion of CdSe nanosheets, attributed to the switching of surface electronic transition dipole moments on the chiral CdSe surface (Fig. 21).127 This study underscores the critical role of surface electronic transitions in modulating the chiral properties of QDs, further enhancing our understanding of their chiroptical activity.
 | ||
| Fig. 20 The top view and side view of the optimized model of (a) chiral Pen-modified CdS QDs and (b) contour plot of an empty LUMO+3 molecular orbital. Reproduced with permission from ref. 61. Copyright 2008 American Chemical Society. | ||
 | ||
| Fig. 21 Optimized coordination configurations and calculated EDM array around the chiral center of L-Cys onto the WZ CdSe surface (a) in the absence of oxygen and (b) in the presence of oxygen. Reproduced with permission from ref. 127. Copyright 2024 American Chemical Society. | ||
Due to early limitations in understanding and instrumentation, the aforementioned theoretical computational methods and models have primarily focused on the chiroptical activity of QDs.88,101 When addressing the mechanism of CPL in QDs, these theories can offer insights into the asymmetric behavior of QDs, but they fall short of fully elucidating the polarization generation mechanism in the excited states of the material.128–130 In contrast, theoretical models for small molecules have been extensively developed, allowing for detailed reconstruction of CPL spectra and the determination of excited state polarization information using methods such as TDDFT, DFT, or other models.3,112 However, for inorganic QDs, constructing direct theoretical models to describe the electronic excited states and decode the CPL signal generation remains relatively underdeveloped and warrants further exploration.
5 Applications of CPL in QDs
The integration of size- and composition-tunable photoluminescence, narrow-band emission spectra, and high quantum efficiency with CPL in inorganic QDs opens up transformative avenues across various disciplines. Notably, CPL QDs hold substantial promise in pioneering applications such as 3D displays, optical data storage, anti-counterfeiting technologies, asymmetric synthesis and catalysis, and chiral sensing. As a result, these nanomaterials have garnered significant attention as a focal point of current research. This section delves into the most groundbreaking applications of CPL QDs, highlighting recent advances that underscore their potential and underscore the ongoing research efforts aimed at unlocking their full capabilities.5.1 3D displays
Since the pioneering report of an electroluminescent device utilizing CdSe QDs in 1994,131 the quest for energy-efficient devices has been relentless.132 The development of highly efficient CPL-emitting materials has gained substantial traction due to their potential to enhance luminescence efficiency and generate high-contrast 3D images in displays.133,134 Typically, Quantum Light Emitting Diodes (QLEDs) employed in flat-panel displays rely on a polarizer and a quarter-wave plate to reduce ambient reflectivity and achieve superior image contrast. However, substituting traditional light sources with inorganic QDs that emit CPL with high color purity as a polarized backlight can significantly reduce emission losses, thereby improving the efficiency of LCD devices.135,136 Consequently, advancements in CPL materials and technologies are of paramount importance in the field of optoelectronics. In a notable study, Professor Yingying Duan's team leveraged a colloidal solution of chiral CdSe/CdS quantum rods as the luminescent core to fabricate chiral QD light-emitting diodes (CQLEDs) on indium tin oxide substrates via spin-coating and evaporation techniques.137 These CQLEDs exhibited negative circularly polarized electroluminescent signals at 600 nm with a |gabs| of 2 × 10−4, marking a significant milestone as the first reported CQLEDs based on the chiral assembly of quantum rods with CPL properties.Counterfeit and substandard products pervade many aspects of daily life, posing serious threats to public health and quality of life. As a result, the development of advanced anti-counterfeiting materials has garnered significant attention.138–140 Chiral functional materials with large glum values have recently emerged as promising candidates due to their unique multimodal optical properties, including fluorescence and CPL, which can be harnessed in anti-counterfeiting technologies to encode concealed information. In a notable study, Professor Taotao Zhuang's team developed a sophisticated multimodal security architecture for information encoding by integrating CPL QDs with other optical features. The team fabricated a smart tag by weaving QD-infused fiber composites into a textile, which exhibits distinctive color changes under varying temperatures or light excitation.141 When viewed through a circularly polarized filter or exposed to light, the QD fiber composites can switch between bright green and transparent states. This innovative textile, endowed with CPL properties, can be controlled externally to generate 3D displays. Moreover, the material's versatile responsiveness to different stimuli enables advanced anti-counterfeiting applications, where displayed information can be dynamically altered by adjusting external conditions (Fig. 22). Moreover, our team has synthesized CPL CdSe@ZnS QDs using a water–oil interface method and fabricated QDs films via the Langmuir–Blodgett (LB) technique. These films exhibited a glum value of approximately 9 × 10−3. When viewed through specific circular polarizers, the L- and D-type chiral QD films demonstrated distinct fluorescence contrasts.98 By integrating 3D printing technology, we achieved diverse QDs chiral configurations within the storage and positioning areas of QR codes. Under UV illumination, the QR codes, used for information storage, were clearly visible and could be seamlessly scanned using a smartphone. However, selectively reducing the brightness of the chiral QDs in the positioning areas rendered the QR codes difficult to discern, thwarting any attempts at forgery or tampering. This dual functionality not only enhances information storage but also strengthens optical anti-counterfeiting measures (Fig. 23). Similarly, Professor Thi Bich Vu's team employed thioglycolic acid (TGA) as a ligand, and with an increase in TGA/Zn2+ ratios, they facilitated the transition of ZnS:Mn2+ QDs from a sphalerite to a wurtzite structure.142 When these ZnS:Mn2+ QDs were applied in anti-counterfeiting ink and written on glass plates, they emitted an orange-red color under 385 nm UV light excitation. These findings are poised for applications in secure anti-counterfeiting and optoelectronic devices.
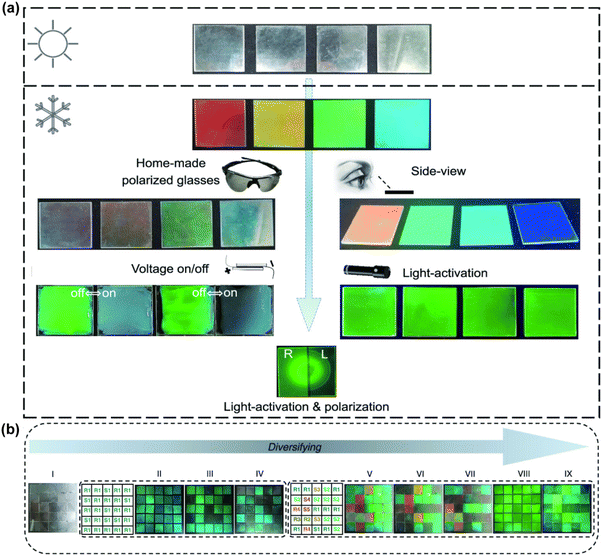 | ||
| Fig. 22 (a) Stimulus-responsive color-changing behavior of multimodal-responsive CPL security materials. (b) Information encryption and anti-counterfeiting design using ultraviolet and polarized light irradiation. Reproduced with permission from ref. 141. Copyright 2023 American Chemical Society. | ||
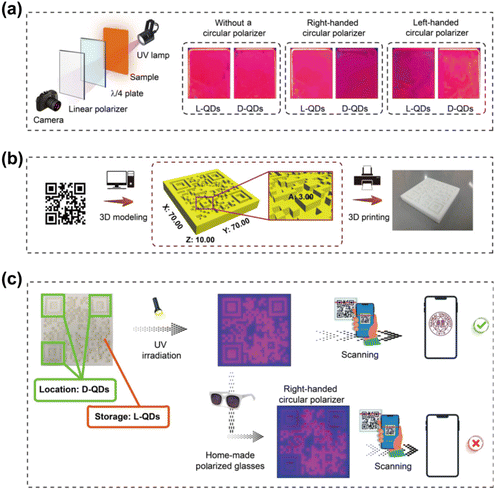 | ||
| Fig. 23 (a) Schematic illustration of the characterization of CPL of chiral CdSe@ZnS nanofilms. (b) Fabrication of QR code models prepared by 3D printing. (c) The multilevel anticounterfeiting of chiral CdSe@ZnS QDs. Reproduced with permission from ref. 98. Copyright 2023 American Chemical Society. | ||
5.2 Asymmetric synthesis/catalysis
Asymmetric catalytic synthesis, a powerful method for producing enantiomerically pure compounds, typically requires only a small amount of a chiral catalyst for chiral amplification or chiral multiplication.143–146 Like ordinary catalysts, most chiral catalysts accelerate the reaction by lowering the free energy of the transition state of the chemical reaction. The key difference is that chiral catalysts allow the reaction to proceed in a direction that favors the generation of mirror-image isomers that match the chirality of the catalyst, thereby controlling the chirality. Chiral inorganic nanomaterials can be synthesized as both chiral carriers and catalytic centers that not only with enantioselective catalytic performances like natural enzymes, but also with significant desirable properties, including convenience in construction and storage, catalytic efficiency, structural stability, and economic performance.147,148 However, a significant challenge arises at the conclusion of the reaction, where the separation of the chiral catalyst from the reaction mixture can be difficult, leading to increased production costs and potential issues with the purity of the final product. To address this issue, ZnS QDs with L-proline-induced chirality were employed as a catalyst in the direct asymmetric hydroxyaldol condensation reaction involving aldehydes and acetone, where acetone functioned as both solvent and reactant (Fig. 24a).149 FTIR suggests monodentate coordination of L-proline with the ZnS surface. Surface zinc ions facilitate the catalytic activity of L-proline by acting as Lewis acids and providing coordination sites for the leaving group. The ZnS nanocrystals facilitated the selective formation of (R)-β-hydroxycarbonyl compounds at ambient temperature, with the reaction efficiently restricted to the acetalization stage. Notably, the ZnS catalysts could be recovered and reused multiple times without any significant loss of catalytic activity, highlighting their potential for sustainable and cost-effective applications.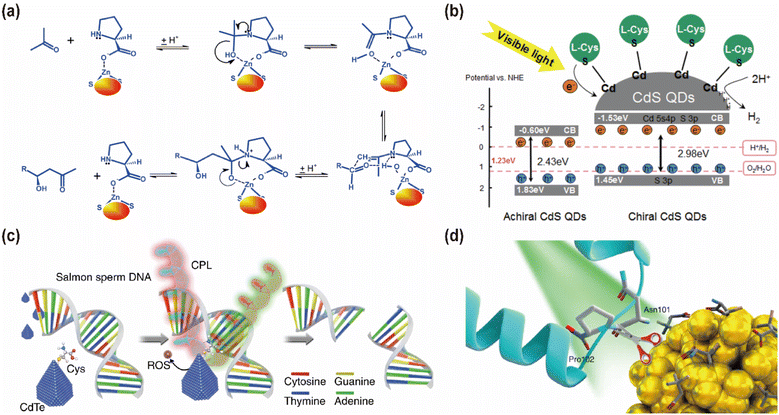 | ||
| Fig. 24 (a) Reaction mechanism of L-proline-induced chiral ZnS QDs as a catalyst. Reproduced with permission from ref. 147. Copyright 2013 Royal Society of Chemistry. (b) H2 evolution mechanism of Chiral CdS QDs. Reproduced with permission from ref. 53. Copyright 2023 Elsevier. (c) Schematic illustration of chiral CdTe-based specific DNA cleavage under CPL irradiation. Reproduced with permission from ref. 50. Copyright 2018 Springer Nature. (d) Schematic illustration of chiral Cu1.96S NPs selectively cleaving capsid proteins under sunlight. Reproduced with permission from ref. 54. Copyright 2022 Springer Nature. | ||
Additionally, QDs with suitable band gaps and specific surfaces are versatile materials capable of catalyzing photocatalytic reactions by harnessing photoinduced electrons and holes. Professor Emily A. Weiss's team demonstrated that CdSe QDs can serve as visible-light chromophores, photocatalysts, and reusable scaffolds for homo- and hetero-intermolecular [2 + 2] photocycloadditions of 4-vinylbenzoic acid derivatives, including aryl-conjugated alkenes, achieving up to 98% switchable regioselectivity and 98% diastereoselectivity for the syn-cyclobutane products.10 The CdSe QDs facilitated catalysis by donating energy to the triplet states of organic molecules from triplet-like excitonic ‘dark’ states. The precisely controlled triplet energy levels of the QD photocatalysts facilitate efficient and selective heterocoupling. This approach presents a strategy for the extrinsic control of cyclobutane product configuration, offering significant potential for reactions proceeding through a triplet excited state.
CPL, as a distinct form of light, can enhance light absorption efficiency, thereby accelerating or even initiating certain photocatalytic reactions.150–152 For instance, chiral CdS QDs exhibit significantly higher photocatalytic hydrogen production activity compared to their non-chiral counterparts under similar condition (Fig. 24b).53 This enhancement is attributed to the introduction of chirality in CdS QDs, which increases both the wide bandgap and conduction band positions, improving the photogenerated electron reduction capability, surface defects, and overall stability. Additionally, chirality in CdS QDs increases the electron density and photoelectron utilization efficiency at the active site while suppressing light-induced electron–hole recombination, thereby enhancing the photocatalytic hydrogen precipitation capacity. Furthermore, QDs capped with chiral ligands have demonstrated efficacy in the photodegradation of various organic compounds.153–155 For example, under visible light irradiation, L-arginine-capped CdS QDs effectively degraded methylene blue and rhodamine B.155 This degradation process is initiated when visible light excites electrons from the valence band to the conduction band in CdS QDs, leading to the formation of electron–hole pairs on the QD surface. These pairs, with their high oxidation potential, oxidize the dyes adsorbed on the QD surface, suggesting that CdS QDs hold promise as potential photocatalysts for the effective treatment of organic pollutants under visible light irradiation.
Moreover, chiral QDs have proven effective in performing specific cleavage of DNA or proteins. For example, chiral CdTe QDs with unique truncated tetrahedral shapes exhibit artificial restriction endonuclease properties when exposed to CPL (Fig. 24c).50 These QDs can selectively recognize and cleave the enzymatic site of the GATATC sequence in double-stranded DNA. The chiral ligands of these QDs show strong affinity for specific DNA sequences, leading to sequence-specific selectivity and changes in CD signaling as the nanoparticles undergo morphological changes. Remarkably, these CdTe QDs have demonstrated the ability to cleave DNA in vivo within living cells and nude mice, indicating favorable biocompatibility. This pioneering research represents the first instance of utilizing chiral inorganic nanoparticles as artificial nucleic acid endonucleases. Additionally, chiral copper sulfide QDs prepared in the presence of chiral L-/D-3-mercapto-glutamic acid have been employed for protein cleavage under CPL irradiation.156 Interestingly, it was observed that L-type QDs exhibit superior catalytic performance under left CPL irradiation, while D-type QDs functioned better under right CPL irradiation. Similar to the CdTe QDs, the mechanism of protein cleavage involves the generation of hydroxyl radicals under CPL irradiation. Further research revealed that light of a given chirality activated a greater number of QDs with matching chirality than the opposite, leading to an increased number of hot electrons and, consequently, hydroxyl radicals.
In a related study, Professor Hua Kuang's team discovered that chiral Cu1.96S nanoparticles (NPs), capped with D-Pen, could site-selectively cleave the capsid of tobacco mosaic virus under sunlight (Fig. 24d).54 Illumination with green light led to polarization-dependent, protease-like hydrolysis of the amide bond between Asn 101 and Pro 102. Simultaneously, these NPs can serve as effective photoactivated antiviral agents for plants. This research not only expands the application of chiral QDs in precision molecular cleavage but also sheds light on the potential role of CPL in enhancing catalytic activity.
5.3 Chiral sensing
The precise identification and quantification of chiral substances are crucial for advancing chiral synthesis,157–159 toxicological assessments,160–162 and clinical diagnostics.163,164 Numerous studies have leveraged chiral QDs to achieve enantiomeric recognition and separation.165–167 Professor Xiaoqing Chen's group developed a chiral fluorescence sensing platform by modifying the surface of CdSe/ZnS QDs with pyroglutamic acid derivatives, facilitating the fluorescent detection of chiral molecules (Fig. 25a).90 This sensor exhibited exceptional selectivity for L-enantiomers, including His, glutamic acid (Glu), and dihydroxyphenylalanine (Dopa), which was attributed to the differential hydrogen bonding interactions between the surface-bound pyroglutamic acid derivatives and the respective enantiomers. Expanding on this concept, Professor Weili Wei's team covalently modified MoS2 and WS2 QDs with chiral ligands Cys and Pen, demonstrating enantioselective peroxidase-like activity in the presence of copper ions (Fig. 25b).168 Their findings revealed the high binding affinity of L-tyrosine for L-Cys QDs, while D-tyrosine exhibited stronger affinity for D-Cys QDs, leading to pronounced enantioselectivity in the photoexcitation of homochiral mixtures, with the enantioselectivity factor reaching up to 6.77.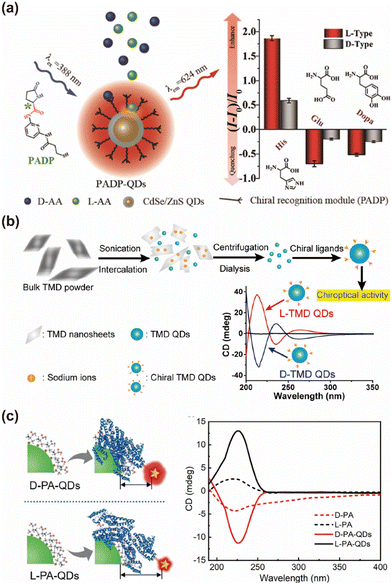 | ||
| Fig. 25 (a) L-Pyroglutamic acid-modified CdSe/ZnS QDs chiral sensing platform for chiral recognition. Reproduced with permission from ref. 90. Copyright 2020 American Chemical Society. (b) Enantioselective catalysis of chiral transition-metal dichalcogenide QDs. Reproduced with permission from ref. 168. Copyright 2018 American Chemical Society. (c) Schematics for the FRET of chiral InP@ZnS QDs. Reproduced with permission from ref. 51. Copyright 2020 Wiley-VCH. | ||
Moreover, substantial research has focused on the modulation of CD signals in response to specific targets.169–171 For instance, professor Li Shang's group elucidated the differential affinities and adsorption orientations of human serum albumin on the surfaces of D- and L-Pen capped InP/ZnS QDs through quantitative fluorescence resonance energy transfer (FRET) analysis (Fig. 25c).51 Complementary CD spectroscopy confirmed significant conformational changes upon interaction with these chiral QDs, validating the observed differences in adsorption behaviors. Similarly, Professor Wittaya Ngeontae's team pioneered the development of a glucose biosensor using chiral CdS QDs as a sensing probe, leveraging CD spectroscopy to monitor the enzymatic production of H2O2 by glucose oxidase (GOx) in response to glucose.172 This reaction etches the chiral CdS QDs, causing a linear decrease in the CD signal that correlates with glucose concentration. The sensor demonstrated a linear detection range of 50–250 μM and a limit of detection (LOD) of 31 μM.
In addition to these applications, QDs have also been employed for the enantiomeric discrimination and enrichment of chiral drugs and biomolecules.173–175 Professor Julia Pérez-Prieto's group utilized CdSe/ZnS QDs capped with methyl ester N-acetyl-L-cysteine to discriminate between chiral 2-arylpropionic acid drugs (2-APAs).173 The emission of QDs was quenched by the drugs in a concentration-dependent manner, enabling the differentiation and quantification of R- and S-enantiomers based on spectral variations. Furthermore, Professor Guangjun Nie's team explored the cytotoxicity and autophagy-inducing effects of chiral GSH-capped CdTe QDs in HepG2 cells, noting that L-GSH-QDs induced greater autophagy and cytotoxicity compared to their D-form counterparts.176 Professor Haibing Li's team developed highly fluorescent and selectively chiral-responsive liquid QDs, termed (S)-1810-QDs, by exploiting the hydrophobic interactions between chiral chains and OA-stabilized QDs.86 These QDs exhibited significant fluorescence and selective chiral responsiveness toward (1R,2S)-2-amino-1,2-diphenylethanol, with a highly chiral-selective response. Moreover, (S)-1810-QDs were fabricated into chiral membranes for the adsorptive separation of (1S,2R)-2-amino-1,2-diphenylethanol and (1R,2S)-2-amino-1,2-diphenylethanol from racemic solutions.
6 Conclusion and prospects
In this review, we made an in-depth examination of the current state of CPL in inorganic QDs, covering their synthesis methodologies, modulation of chiroptical activity, mechanistic insights, and emerging applications. The review highlights the nuanced interplay between synthetic approaches—such as direct aqueous synthesis, post-synthetic modifications, and interface-mediated methods—and their influence on the resultant CPL properties. We discussed the impact of factors such as the ligand type, QD size, shell architecture, and interaction modes on chiroptical activity, underscoring the importance of these parameters in optimizing CPL signals. Additionally, we summarized the theoretical approaches employed to understand the chiroptical activity of QDs, including TDDFT, DDA, NDCO models, DFT, and AIMD. These methods offer critical insights into the mechanisms underlying CPL in QDs, although gaps remain in comprehensively modeling the excited-state behaviors responsible for CPL. The review concludes with a detailed exploration of potential applications, such as 3D displays, optical data storage, asymmetric catalysis, and chiral sensing.Despite significant advancements, the field of CPL in QDs remains in its early stages. The current glum values in QDs, ranging from 10−5 to 10−2, are notably lower than those achieved in organic molecules or supramolecular systems, which can reach up to ±2.177 Furthermore, the chiral functionalization processes often lead to irreversible reductions in quantum yield, posing a significant barrier to the practical application of CPL QDs.33 While interface-mediated synthesis methods have shown potential in addressing the trade-off between CPL asymmetry and luminescence intensity, there remains substantial room for improvement in material properties. Enhancing both CPL asymmetry and quantum yield presents a formidable challenge for synthetic chemists and photophysics researchers. According to Rosenfeld's theory, manipulating the balance between electric and magnetic dipole interactions offers a viable strategy for tuning CPL properties. This could be achieved by doping with paramagnetic materials, introducing highly electronegative elements, or adjusting ligands to alter the electronic density of the QDs.22 While this approach has been validated in chiral small molecules,122 supramolecular assemblies,114 and metal clusters, its efficacy in QDs is still under investigation, necessitating further exploration and empirical validation.
The mechanistic understanding of CPL in inorganic QDs is currently limited by a predominant reliance on chiroptical spectral analyses, which primarily address the differences in polarized absorption rather than the distinct electronic processes underlying polarized emission from excited states. While there may be some correlation between these phenomena, they represent fundamentally different physical processes. This highlights a significant gap in both theoretical modeling and spectral simulation, underscoring the need for more advanced and specialized approaches to accurately interpret and predict CPL behavior in QDs. Recently, in the study of perovskite QDs, researchers have begun employing circularly polarized femtosecond transient absorption spectroscopy to compare the differences in electronic transitions for various spin states.130 This approach seeks to establish a clearer connection between CPL characteristics and the electronic states of the material, thereby enhancing our understanding of the underlying mechanisms driving CPL signals in these systems.178 Although the structural and energy level complexities of inorganic QDs present additional challenges compared to perovskites, these new methodologies offer promising avenues for deepening our mechanistic insights into CPL in QDs.
Moving forward, substantial efforts are needed to enhance the performance of CPL QDs, particularly in achieving higher glum values and ensuring the scalability and stability of these materials for real-world applications. The self-assembly of QDs with chiral templates, such as hydrogels, polymers, and liquid crystals, has shown potential for producing high-quality CPL materials. However, to fully leverage these materials in diverse applications—ranging from 3D displays and optical storage to asymmetric catalysis and chiral sensing—further refinement at the fundamental level of CPL QDs is essential. Additionally, the potential of CPL QDs in emerging fields such as covert information transmission, spintronic information transfer, and polarized communication warrants deeper exploration. Addressing these challenges will be crucial for advancing the understanding and application of CPL QDs, thereby unlocking their full potential in both scientific research and technological innovation.
Data availability
In this review manuscript, no primary research results, software or code have been included. No new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22293030, 22293032, 91859123, 22474064 and 22204082), the National Key Research and Development Program (Grant No. 2019YFA0210100), the National Postdoctoral Program for Innovative Talents (Grant No. BX20220156), the China Postdoctoral Science Foundation (Grant No. 2023M731789), the ‘Frontiers Science Center for New Organic Matter’, Nankai University (Grant No. 63181206), and the Haihe Laboratory of Sustainable Chemical Transformations.References
- H. Tanaka, Y. Inoue and T. Mori, ChemPhotoChem, 2018, 2, 386–402 CrossRef CAS.
- E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem. – Eur. J., 2015, 21, 13488–13500 CrossRef PubMed.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Matter, 2022, 5, 837–875 CrossRef CAS.
- S. Liu, X. Liu, Y. Wu, D. Zhang, Y. Wu, H. Tian, Z. Zheng and W.-H. Zhu, Matter, 2022, 5, 2319–2333 CrossRef CAS.
- X. Zhan, F.-F. Xu, Z. Zhou, Y. Yan, J. Yao and Y. S. Zhao, Adv. Mater., 2021, 33, 2104418 CrossRef CAS.
- M. Schadt, Annu. Rev. Mater. Sci., 1997, 27, 305–379 CrossRef CAS.
- Z.-L. Gong, X. Zhu, Z. Zhou, S.-W. Zhang, D. Yang, B. Zhao, Y.-P. Zhang, J. Deng, Y. Cheng, Y.-X. Zheng, S.-Q. Zang, H. Kuang, P. Duan, M. Yuan, C.-F. Chen, Y. S. Zhao, Y.-W. Zhong, B. Z. Tang and M. Liu, Sci. China: Chem., 2021, 64, 2060–2104 CrossRef CAS.
- Y. Shi, J. Han, X. Jin, W. Miao, Y. Zhang and P. Duan, Adv. Sci., 2022, 9, 2201565 CrossRef CAS.
- F. Zinna, U. Giovanella and L. D. Bari, Adv. Mater., 2015, 27, 1791–1795 CrossRef CAS PubMed.
- Y. Jiang, C. Wang, C. R. Rogers, M. S. Kodaimati and E. A. Weiss, Nat. Chem., 2019, 11, 1034–1040 CrossRef CAS.
- W. Ma, J. Mao and C. Hao, Appl. Catal., B, 2019, 245, 691–697 CrossRef CAS.
- S. Li, J. Liu, N. S. Ramesar, H. Heinz, L. Xu, C. Xu and N. A. Kotov, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- Y. Imai, Y. Nakano, T. Kawai and J. Yuasa, Angew. Chem., Int. Ed., 2018, 57, 8973–8978 CrossRef CAS.
- Q. Zhao, W. Zhu, W. Cai, J. Li, D. Wu and Y. Kong, Anal. Chem., 2022, 94, 9399–9406 CrossRef CAS.
- C. Han and H. Li, Small, 2008, 4, 1344–1350 CrossRef CAS.
- F. P. Milton, J. Govan, M. V. Mukhina and Y. K. Gun'ko, Nanoscale Horiz., 2015, 1, 14–26 RSC.
- S. Lin, Y. Tang, W. Kang, H. K. Bisoyi, J. Guo and Q. Li, Nat. Commun., 2023, 14, 3005 CrossRef CAS PubMed.
- Y. Hu, Z. Huang, I. Willner and X. Ma, CCS Chem., 2023, 6, 518–527 CrossRef.
- J. F. Sherson, H. Krauter, R. K. Olsson, B. Julsgaard, K. Hammerer, I. Cirac and E. S. Polzik, Nature, 2006, 443, 557–560 CrossRef CAS PubMed.
- N. Liang, C. Cao, Z. Xie, J. Liu, Y. Feng and C.-J. Yao, Mater. Today, 2024, 75, 309–333 CrossRef CAS.
- H. Shigemitsu, K. Kawakami, Y. Nagata, R. Kajiwara, S. Yamada, T. Mori and T. Kida, Angew. Chem., Int. Ed., 2022, 61, e202114700 CrossRef CAS.
- J. Zhang, L. Dai, A. M. Webster, W. T. K. Chan, L. E. Mackenzie, R. Pal, S. L. Cobb and G.-L. Law, Angew. Chem., Int. Ed., 2021, 60, 1004–1010 CrossRef CAS PubMed.
- Z.-P. Yan, T.-T. Liu, R. Wu, X. Liang, Z.-Q. Li, L. Zhou, Y.-X. Zheng and J.-L. Zuo, Adv. Funct. Mater., 2021, 31, 2103875 CrossRef CAS.
- L. Xu, B.-R. Gao, X.-H. Xu, L. Zhou, N. Liu and Z.-Q. Wu, Angew. Chem., Int. Ed., 2022, 61, e202204966 CrossRef CAS.
- S. Lee, K. Y. Kim, S. H. Jung, J. H. Lee, M. Yamada, R. Sethy, T. Kawai and J. H. Jung, Angew. Chem., Int. Ed., 2019, 58, 18878–18882 CrossRef CAS.
- J. Roose, B. Z. Tang and K. S. Wong, Small, 2016, 12, 6495–6512 CrossRef CAS PubMed.
- F. Pop, N. Zigon and N. Avarvari, Chem. Rev., 2019, 119, 8435–8478 Search PubMed.
- S. Ma, J. Ahn and J. Moon, Adv. Mater., 2021, 33, 2005760 CrossRef CAS PubMed.
- M.-M. Zhang, K. Li and S.-Q. Zang, Adv. Opt. Mater., 2020, 8, 1902152 CrossRef CAS.
- X. Yang, X. Jin, A. Zheng and P. Duan, ACS Nano, 2023, 17, 2661–2668 CrossRef CAS.
- J. Yuan, X. Lu and Q. Lu, Aggregate, 2024, 5, e431 CrossRef CAS.
- Y. Wu, M. Li, Z. Zheng, Z.-Q. Yu and W.-H. Zhu, J. Am. Chem. Soc., 2023, 145, 12951–12966 CrossRef CAS PubMed.
- X. Gao, B. Han, X. Yang and Z. Tang, J. Am. Chem. Soc., 2019, 141, 13700–13707 CrossRef CAS PubMed.
- L. Lin, A.-A. Liu, W. Zhao, Y. Yang, D.-L. Zhu, B.-R. Dong, F. Ding, D. Ning, X. Zhu, D. Liu and D.-W. Pang, J. Am. Chem. Soc., 2024, 146, 21348–21356 CrossRef.
- M. J. Natan, J. W. Thackeray and M. S. Wrighton, J. Phys. Chem., 1986, 90, 4089–4098 CrossRef CAS.
- M. C. Brelle, J. Z. Zhang, L. Nguyen and R. K. Mehra, J. Phys. Chem. A, 1999, 103, 10194–10201 CrossRef CAS.
- M. P. Moloney, Y. K. Gun'ko and J. M. Kelly, Chem. Commun., 2007, 3900–3902 RSC.
- U. Tohgha, K. Varga and M. Balaz, Chem. Commun., 2013, 49, 1844–1846 CAS.
- S. A. Gallagher, M. P. Moloney, M. Wojdyla, S. J. Quinn, J. M. Kelly and Y. K. Gun'ko, J. Mater. Chem., 2010, 20, 8350–8355 RSC.
- J. K. Choi, B. E. Haynie, U. Tohgha, L. Pap, K. W. Elliott, B. M. Leonard, S. V. Dzyuba, K. Varga, J. Kubelka and M. Balaz, ACS Nano, 2016, 10, 3809–3815 CAS.
- Z. Li, W. Li, D. Li, W. Tang, H. Liang, H. Song, C. Chen, L. Gao and J. Tang, Front. Optoelectron., 2024, 17, 1–7 Search PubMed.
- L. Branzi, F. Purcell-Milton, C. Cressoni, M. Back, E. Cattaruzza, A. Speghini, Y. K. Gun'ko and A. Benedetti, Nanoscale, 2022, 14, 12174–12182 RSC.
- H. Nakamura, W. Kato, M. Uehara, K. Nose, T. Omata, S. Otsuka-Yao-Matsuo, M. Miyazaki and H. Maeda, Chem. Mater., 2006, 18, 3330–3335 CrossRef CAS.
- M. Ueda, Z. L. Goo, K. Minami, N. Yoshinari and T. Konno, Angew. Chem., Int. Ed., 2019, 58, 14673–14678 CrossRef CAS.
- S. Parzyszek, J. Tessarolo, A. Pedrazo-Tardajos, A. M. Ortuño, M. Bagiński, S. Bals, G. H. Clever and W. Lewandowski, ACS Nano, 2022, 16, 18472–18482 CrossRef CAS PubMed.
- K. Varga, S. Tannir, B. E. Haynie, B. M. Leonard, S. V. Dzyuba, J. Kubelka and M. Balaz, ACS Nano, 2017, 11, 9846–9853 CrossRef CAS PubMed.
- J. Cheng, J. Hao, H. Liu, J. Li, J. Li, X. Zhu, X. Lin, K. Wang and T. He, ACS Nano, 2018, 12, 5341–5350 CrossRef CAS.
- X. Gao, X. Zhang, K. Deng, B. Han, L. Zhao, M. Wu, L. Shi, J. Lv and Z. Tang, J. Am. Chem. Soc., 2017, 139, 8734–8739 CrossRef CAS.
- J. Hao, Y. Li, J. Miao, R. Liu, J. Li, H. Liu, Q. Wang, H. Liu, M.-H. Delville, T. He, K. Wang, X. Zhu and J. Cheng, ACS Nano, 2020, 14, 10346–10358 CrossRef CAS PubMed.
- M. Sun, L. Xu, A. Qu, P. Zhao, T. Hao, W. Ma, C. Hao, X. Wen, F. M. Colombari, A. F. de Moura, N. A. Kotov, C. Xu and H. Kuang, Nat. Chem., 2018, 10, 821–830 CrossRef CAS.
- S. Qu, F. Sun, Z. Qiao, J. Li and L. Shang, Small, 2020, 16, 1907633 CrossRef CAS PubMed.
- G. Li, X. Zhang, X. Fei, J. Li, H. Liu, W. Liu, Y. Yang, B. Li, M. Liu, G. Yang and T. Zhang, ACS Nano, 2022, 16, 12991–13001 CrossRef CAS.
- S. Zhang, S. Sun, L. Song and E. Liu, Int. J. Hydrogen Energy, 2023, 48, 33903–33912 CrossRef CAS.
- R. Gao, L. Xu, M. Sun, M. Xu, C. Hao, X. Guo, F. M. Colombari, X. Zheng, P. Král, A. F. de Moura, C. Xu, J. Yang, N. A. Kotov and H. Kuang, Nat. Catal., 2022, 5, 694–707 CAS.
- T. Wu, J. Kapitán, V. Mašek and P. Bouř, Angew. Chem., Int. Ed., 2015, 54, 14933–14936 CrossRef CAS.
- A. Rodger, K. Venkatesan, J. R. Aldrich-Wright, C. Brodie and A. E. Garcia-Bennett, Anal. Chem., 2024, 96, 3810–3816 CrossRef CAS PubMed.
- S. Huo, P. Duan, T. Jiao, Q. Peng and M. Liu, Angew. Chem., Int. Ed., 2017, 56, 12174–12178 CrossRef CAS.
- C. Li, X. Jin, T. Zhao, J. Zhou and P. Duan, Nanoscale Adv., 2019, 1, 508–512 RSC.
- Y.-Y. Zhao, H. Luo, Q. Ge, M. Liu, Z. Tao and H. Cong, Sens. Actuators, B, 2021, 336, 129750 CrossRef CAS.
- X. Wang, L. Zhao, C. Wang, X. Feng, Q. Ma, G. Yang, T. Wang, X. Yan and J. Jiang, Small, 2022, 18, 2104438 CrossRef CAS.
- S. D. Elliott, M. P. Moloney and Y. K. Gun'ko, Nano Lett., 2008, 8, 2452–2457 CrossRef CAS PubMed.
- F. Purcell-Milton, A. K. Visheratina, V. A. Kuznetsova, A. Ryan, A. O. Orlova and Y. K. Gun'ko, ACS Nano, 2017, 11, 9207–9214 CrossRef CAS.
- D. R. Baker and P. V. Kamat, Langmuir, 2010, 26, 11272–11276 CrossRef CAS PubMed.
- S. Di Noja, F. Amato, F. Zinna, L. Di Bari, G. Ragazzon and M. Prato, Angew. Chem., Int. Ed., 2022, 61, e202202397 CrossRef CAS PubMed.
- A. Das, E. V. Kundelev, A. A. Vedernikova, S. A. Cherevkov, D. V. Danilov, A. V. Koroleva, E. V. Zhizhin, A. N. Tsypkin, A. P. Litvin, A. V. Baranov, A. V. Fedorov, E. V. Ushakova and A. L. Rogach, Light: Sci. Appl., 2022, 11, 92 CrossRef CAS PubMed.
- Y. Shi, W. Su, Q. Teng, C. Li, T. Yuan, H. Xu, X. Song, Y. Han, S. Wei, Y. Zhang, X. Li, Y. Li, L. Fan and F. Yuan, Matter, 2023, 6, 2776–2806 CrossRef CAS.
- M. P. Moloney, J. Govan, A. Loudon, M. Mukhina and Y. K. Gun'ko, Nat. Protoc., 2015, 10, 558–573 Search PubMed.
- A. Ben-Moshe, A. O. Govorov and G. Markovich, Angew. Chem., Int. Ed., 2013, 52, 1275–1279 CrossRef CAS PubMed.
- M. Naito, K. Iwahori, A. Miura, M. Yamane and I. Yamashita, Angew. Chem., Int. Ed., 2010, 49, 7006–7009 CrossRef CAS.
- P. T. Buz, F. D. Duman, M. Erkisa, G. Demirci, F. Ari, E. Ulukaya and H. Y. Acar, Nanomedicine, 2019, 14, 969–987 Search PubMed.
- F. Yang, G. Gao, J. Wang, R. Chen and T. Sun, J. Colloid Interface Sci., 2019, 537, 422–430 Search PubMed.
- Y. Zhou, M. Yang, K. Sun, Z. Tang and N. A. Kotov, J. Am. Chem. Soc., 2010, 132, 6006–6013 CAS.
- T. Nakashima, Y. Kobayashi and T. Kawai, J. Am. Chem. Soc., 2009, 131, 10342–10343 CAS.
- R. Zhou, K.-Y. Wei, J.-S. Zhao and Y.-B. Jiang, Chem. Commun., 2011, 47, 6362–6364 CAS.
- J. E. Govan, E. Jan, A. Querejeta, N. A. Kotov and Y. K. Gun'ko, Chem. Commun., 2010, 46, 6072–6074 RSC.
- X. Chen, J. L. Hutchison, P. J. Dobson and G. Wakefield, J. Mater. Sci., 2009, 44, 285–292 CrossRef CAS.
- I.-S. Liu, H.-H. Lo, C.-T. Chien, Y.-Y. Lin, C.-W. Chen, Y.-F. Chen, W.-F. Su and S.-C. Liou, J. Mater. Chem., 2008, 18, 675–682 RSC.
- M. M. Krause and P. Kambhampati, Phys. Chem. Chem. Phys., 2015, 17, 18882–18894 RSC.
- A. Veamatahau, B. Jiang, T. Seifert, S. Makuta, K. Latham, M. Kanehara, T. Teranishi and Y. Tachibana, Phys. Chem. Chem. Phys., 2014, 17, 2850–2858 RSC.
- S. V. Kilina, P. K. Tamukong and D. S. Kilin, Acc. Chem. Res., 2016, 49, 2127–2135 CrossRef CAS PubMed.
- C. Giansante and I. Infante, J. Phys. Chem. Lett., 2017, 8, 5209–5215 CrossRef CAS PubMed.
- S. Ghosh, U. Ross, A. M. Chizhik, Y. Kuo, B. G. Jeong, W. K. Bae, K. Park, J. Li, D. Oron, S. Weiss, J. Enderlein and A. I. Chizhik, J. Phys. Chem. Lett., 2023, 14, 2702–2707 CrossRef CAS.
- A. Ben-Moshe, A. Teitelboim, D. Oron and G. Markovich, Nano Lett., 2016, 16, 7467–7473 CrossRef CAS PubMed.
- Y. H. Kwon, Y. A. Joh, B. M. Leonard, M. Balaz and K. Varga, J. Colloid Interface Sci., 2023, 642, 771–778 CrossRef CAS PubMed.
- U. Tohgha, K. K. Deol, A. G. Porter, S. G. Bartko, J. K. Choi, B. M. Leonard, K. Varga, J. Kubelka, G. Muller and M. Balaz, ACS Nano, 2013, 7, 11094–11102 CrossRef CAS.
- J. Zhang, J. Ma, F. Shi, D. Tian and H. Li, Adv. Mater., 2017, 29, 1700296 CrossRef PubMed.
- F. Feizi, M. Shamsipur, A. Barati, M. B. Gholivand and F. Mousavi, Microchem. J., 2020, 158, 105168 CrossRef CAS.
- G. Li, X. Fei, H. Liu, J. Gao, J. Nie, Y. Wang, Z. Tian, C. He, J.-L. Wang, C. Ji, D. Oron and G. Yang, ACS Nano, 2020, 14, 4196–4205 CrossRef CAS.
- M. V. Mukhina, V. G. Maslov, A. V. Baranov, A. V. Fedorov, A. O. Orlova, F. Purcell-Milton, J. Govan and Y. K. Gun'ko, Nano Lett., 2015, 15, 2844–2851 CrossRef CAS PubMed.
- F. Zhu, J. Wang, S. Xie, Y. Zhu, L. Wang, J. Xu, S. Liao, J. Ren, Q. Liu, H. Yang and X. Chen, Anal. Chem., 2020, 92, 12040–12048 CrossRef CAS PubMed.
- Z. Wang, Y. Wang, G. Adamo, J. Teng and H. Sun, Laser Photonics Rev., 2019, 13, 1800276 CrossRef.
- V. Kuznetsova, Y. Gromova, M. Martinez-Carmona, F. Purcell-Milton, E. Ushakova, S. Cherevkov, V. Maslov and Y. K. Gun'ko, Nanophotonics, 2021, 10, 797–824 CrossRef CAS.
- X. Gao, X. Zhang, L. Zhao, P. Huang, B. Han, J. Lv, X. Qiu, S.-H. Wei and Z. Tang, Nano Lett., 2018, 18, 6665–6671 CrossRef CAS.
- M. Puri and V. E. Ferry, ACS Nano, 2017, 11, 12240–12246 CrossRef CAS PubMed.
- X. Shao, Y. Wu, S. Jiang, B. Li, T. Zhang and Y. Yan, J. Mater. Chem. C, 2021, 9, 555–561 RSC.
- I. V. Martynenko, A. S. Baimuratov, V. A. Osipova, V. A. Kuznetsova, F. Purcell-Milton, I. D. Rukhlenko, A. V. Fedorov, Y. K. Gun'ko, U. Resch-Genger and A. V. Baranov, Chem. Mater., 2018, 30, 465–471 CrossRef CAS.
- X. Shao, T. Zhang, B. Li, Y. Wu, S. Li, J. Wang and S. Jiang, Chirality, 2021, 33, 167–175 CrossRef CAS.
- J. Cai, A.-A. Liu, X.-H. Shi, H. Fu, W. Zhao, L. Xu, H. Kuang, C. Xu and D.-W. Pang, J. Am. Chem. Soc., 2023, 145, 24375–24385 CrossRef CAS.
- Y. Wu, X. Shao, Y. Zhou, S. Jiang, T. Zhang and Y. Yan, Nanotechnology, 2021, 32, 375701 CrossRef CAS.
- V. A. Kuznetsova, E. Mates-Torres, N. Prochukhan, M. Marcastel, F. Purcell-Milton, J. O'Brien, A. K. Visheratina, M. Martinez-Carmona, Y. Gromova, M. Garcia-Melchor and Y. K. Gun'ko, ACS Nano, 2019, 13, 13560–13572 CrossRef CAS PubMed.
- S. Jiang and N. A. Kotov, Adv. Mater., 2023, 35, 2108431 CrossRef CAS.
- G. Pacchioni, Nat. Rev. Mater., 2019, 4, 226–226 CrossRef.
- H. Häkkinen, Nat. Chem., 2012, 4, 443–455 CrossRef.
- Y. H. Kwon, S. Tannir, M. Balaz and K. Varga, Chirality, 2022, 34, 70–76 CrossRef CAS PubMed.
- Y. A. Joh, Y. H. Kwon, S. Tannir, B. M. Leonard, J. Kubelka, K. Varga and M. Balaz, J. Mater. Chem. C, 2021, 9, 17483–17495 RSC.
- A. Ben Moshe, D. Szwarcman and G. Markovich, ACS Nano, 2011, 5, 9034–9043 CrossRef CAS.
- X. Shao, T. Zhang, B. Li, M. Zhou, X. Ma, J. Wang and S. Jiang, Inorg. Chem., 2019, 58, 6534–6543 CrossRef CAS.
- H.-G. Kwon, T. Lee, K. Kim, D.-H. Kim, H. Seo, O.-P. Kwon, J. Kwak and S.-W. Kim, Small, 2024, 20, 2304592 CAS.
- Y. Zhang, H. Yang, X. An, Z. Wang, X. Yang, M. Yu, R. Zhang, Z. Sun and Q. Wang, Small, 2020, 16, 2001003 CAS.
- A. K. Visheratina, A. O. Orlova, F. Purcell-Milton, V. A. Kuznetsova, A. A. Visheratin, E. V. Kundelev, V. G. Maslov, A. V. Baranov, A. V. Fedorov and Y. K. Gun'ko, J. Mater. Chem. C, 2018, 6, 1759–1766 CAS.
- W. Lin, Y. Niu, R. Meng, L. Huang, H. Cao, Z. Zhang, H. Qin and X. Peng, Nano Res., 2016, 9, 260–271 Search PubMed.
- G. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo and S. Abbate, Chirality, 2016, 28, 696–707 Search PubMed.
- C. Zhang, S. Li, X.-Y. Dong and S.-Q. Zang, Aggregate, 2021, 2, e48 Search PubMed.
- H.-Y. Wong, Chem, 2019, 5, 3058–3095 Search PubMed.
- F. Song, Z. Zhao, Z. Liu, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 3284–3301 Search PubMed.
- F. Zinna and L. Di Bari, Chirality, 2015, 27, 1–13 Search PubMed.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2020, 32, 1900110 CrossRef CAS PubMed.
- Y. Kondo, S. Suzuki, M. Watanabe, A. Kaneta, P. Albertini and K. Nagamori, Front. Chem., 2020, 8, 527 CrossRef CAS PubMed.
- F. S. Richardson and J. P. Riehl, Chem. Rev., 1977, 77, 773–792 CrossRef CAS.
- L. Arrico, L. Di Bari and F. Zinna, Chem. – Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed.
- A. Zheng, T. Zhao, X. Jin, W. Miao and P. Duan, Nanoscale, 2022, 14, 1123–1135 RSC.
- S. Sato, A. Yoshii, S. Takahashi, S. Furumi, M. Takeuchi and H. Isobe, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 13097–13101 CrossRef CAS.
- J. Lv, X. Yang and Z. Tang, Adv. Mater., 2023, 35, 2209539 CrossRef CAS PubMed.
- N. Berova, L. D. Bari and G. Pescitelli, Chem. Soc. Rev., 2007, 36, 914–931 RSC.
- A. O. Govorov, Z. Fan, P. Hernandez, J. M. Slocik and R. R. Naik, Nano Lett., 2010, 10, 1374–1382 CrossRef CAS PubMed.
- Y. Zhou, Z. Zhu, W. Huang, W. Liu, S. Wu, X. Liu, Y. Gao, W. Zhang and Z. Tang, Angew. Chem., Int. Ed., 2011, 50, 11456–11459 CrossRef CAS.
- J. Guo, Z. Zhao, R. Sun, Y. Zhou and X. Gao, ACS Appl. Nano Mater., 2024, 7, 16894–16900 CrossRef CAS.
- Y. Han, W. Liang, X. Lin, Y. Li, F. Sun, F. Zhang, P. C. Sercel and K. Wu, Nat. Mater., 2022, 21, 1282–1289 CrossRef CAS.
- P. Odenthal, W. Talmadge, N. Gundlach, R. Wang, C. Zhang, D. Sun, Z.-G. Yu, Z. Valy Vardeny and Y. S. Li, Nat. Phys., 2017, 13, 894–899 Search PubMed.
- X. Lin, Y. Han, J. Zhu and K. Wu, Nat. Nanotechnol., 2023, 18, 124–130 CAS.
- V. L. Colvin, M. C. Schlamp and A. P. Alivisatos, Nature, 1994, 370, 354–357 CrossRef CAS.
- Y. Shang and Z. Ning, Natl. Sci. Rev., 2017, 4, 170–183 CAS.
- Y. Chen, X. Yang and J. Gao, Light: Sci. Appl., 2019, 8, 45 Search PubMed.
- J. Zhao, Y. Yan, Z. Gao, Y. Du, H. Dong, J. Yao and Y. S. Zhao, Nat. Commun., 2019, 10, 870 Search PubMed.
- Z. Liu, C. Zhang, X. Liu, A. Ren, Z. Zhou, C. Qiao, Y. Guan, Y. Fan, F. Hu and Y. S. Zhao, Adv. Sci., 2021, 8, 2102065 CrossRef CAS PubMed.
- M. K. Jana, R. Song, H. Liu, D. R. Khanal, S. M. Janke, R. Zhao, C. Liu, Z. Valy Vardeny, V. Blum and D. B. Mitzi, Nat. Commun., 2020, 11, 4699 CrossRef CAS PubMed.
- T. Duan, J. Ai, S. Chen, G. He, X. Guo, L. Han, S. Che and Y. Duan, Nano Res., 2022, 15, 9573–9577 CrossRef CAS.
- N. Vahedigharehchopogh, O. Kıbrıslı, E. Erol, M. Ç. Ersundu and A. E. Ersundu, J. Mater. Chem. C, 2021, 9, 2037–2046 RSC.
- L. A. de Azevedo, A. Gamonal, R. Maier-Queiroz, C. S. Silva, J. N. S. Ferro, P. d'Amorim, S. C. Oliveira, E. O. Barreto, L. L. da Luz and S. A. Júnior, J. Mater. Chem. C, 2021, 9, 9261–9270 RSC.
- P. R. Anusuyadevi, R. Shanker, Y. Cui, A. V. Riazanova, M. Järn, M. P. Jonsson and A. J. Svagan, Adv. Mater., 2021, 33, 2101519 CrossRef CAS PubMed.
- Q. Guo, M. Zhang, Z. Tong, S. Zhao, Y. Zhou, Y. Wang, S. Jin, J. Zhang, H.-B. Yao, M. Zhu and T. Zhuang, J. Am. Chem. Soc., 2023, 145, 4246–4253 CrossRef CAS.
- T. P. Nguyen, T. P. Nguyen, Q. V. Lam and T. B. Vu, Solid State Sci., 2020, 101, 106123 CrossRef CAS.
- G. Yang and J. Wang, Angew. Chem., Int. Ed., 2024, e202412805 CAS.
- B. Han, Y. Li, Y. Yu and L. Gong, Nat. Commun., 2019, 10, 3804 CrossRef PubMed.
- X. Liu and R.-H. Jin, Chem. Synth., 2021, 1, 14 Search PubMed.
- Y.-P. Xue, C.-H. Cao and Y.-G. Zheng, Chem. Soc. Rev., 2018, 47, 1516–1561 RSC.
- N. A. Kotov, L. M. Liz-Marzán and Q. Wang, Mater. Adv., 2022, 3, 3677–3679 RSC.
- S. Qu, Q. Jia, Z. Li, Z. Wang and L. Shang, Sci. Bull., 2022, 67, 1274–1283 CrossRef CAS.
- E. Shah and H. P. Soni, RSC Adv., 2013, 3, 17453–17461 RSC.
- W. Liu, X. Wang, X. Fang, X. Ju, D. Wang and J. Wang, Chem. Eng. J., 2024, 495, 153549 CrossRef CAS.
- L. Xu, M. Sun, P. Cheng, R. Gao, H. Wang, W. Ma, X. Shi, C. Xu and H. Kuang, Adv. Funct. Mater., 2018, 28, 1707237 CrossRef.
- C. Hao, L. Xu, W. Ma, X. Wu, L. Wang, H. Kuang and C. Xu, Adv. Funct. Mater., 2015, 25, 5816–5822 CrossRef CAS.
- M. Ai, L. Pan, C. Shi, Z.-F. Huang, X. Zhang, W. Mi and J.-J. Zou, Nat. Commun., 2023, 14, 4562 Search PubMed.
- X. Yue, S. Li, H. Lin, C. Xu and L. Xu, Adv. Funct. Mater., 2023, 33, 2210046 CrossRef CAS.
- Y. Guo, L. Wang, L. Yang, J. Zhang, L. Jiang and X. Ma, Mater. Lett., 2011, 65, 486–489 Search PubMed.
- C. Hao, R. Gao, Y. Li, L. Xu, M. Sun, C. Xu and H. Kuang, Angew. Chem., Int. Ed., 2019, 58, 7371–7374 CrossRef CAS PubMed.
- H. Kuang, C. Xu and Z. Tang, Adv. Mater., 2020, 32, 2005110 CrossRef CAS.
- Y. Duan and S. Che, Adv. Mater., 2023, 35, 2205088 Search PubMed.
- T. Li, Y. Pan, L. Ding, Y. Kang, X.-Q. Hao, Y. Guo and L. Shi, Chem. Synth., 2024, 4, 35 Search PubMed.
- X. Wang, Chem, 2017, 2, 749–750 CAS.
- M. Pilz, P. Cavelius, F. Qoura, D. Awad and T. Brück, Biotechnol. Adv., 2023, 67, 108210 CrossRef CAS PubMed.
- G. Wang, H. T. Ang, S. R. Dubbaka, P. O'Neill and J. Wu, Trends Chem., 2023, 5, 432–445 CrossRef CAS.
- W. Chen, L. Fu, Z. Zhu, J. Liu, L. Cheng, Z. Xu, H. Dong, J. Ma, Y. Li and X. Fan, Chin. Chem. Lett., 2023, 34, 107713 CrossRef CAS.
- H. Caner, E. Groner, L. Levy and I. Agranat, Drug Discovery Today, 2004, 9, 105–110 CrossRef CAS.
- S. Zare and J. Tashkhourian, Microchim. Acta, 2019, 187, 71 CrossRef.
- F. Gao, S. Ma, X. Xiao, Y. Hu, D. Zhao and Z. He, Talanta, 2017, 163, 102–110 CrossRef CAS PubMed.
- C. Carrillo-Carrión, S. Cárdenas, B. M. Simonet and M. Valcárcel, Anal. Chem., 2009, 81, 4730–4733 CrossRef.
- H. Zhang, H. He, X. Jiang, Z. Xia and W. Wei, ACS Appl. Mater. Interfaces, 2018, 10, 30680–30688 CrossRef CAS.
- W. Tedsana, T. Tuntulani and W. Ngeontae, Anal. Chim. Acta, 2015, 867, 1–8 CrossRef CAS.
- K. Ngamdee, K. Chaiendoo and C. Saiyasombat, Spectrochim. Acta, Part A, 2019, 211, 313–321 CrossRef CAS PubMed.
- P. Sianglam, S. Kulchat and T. Tuntulani, Spectrochim. Acta, Part A, 2017, 183, 408–416 CrossRef CAS PubMed.
- K. Ngamdee and W. Ngeontae, Sens. Actuators, B, 2018, 274, 402–411 CrossRef CAS.
- T. Delgado-Pérez, L. M. Bouchet, M. de la Guardia, R. E. Galian and J. Pérez-Prieto, Chem. – Eur. J., 2013, 19, 11068–11076 CrossRef PubMed.
- S. R. Ahmed and S. Neethirajan, Global Chall., 2018, 2, 1700071 CrossRef PubMed.
- I. V. Martynenko, V. A. Kuznetsova, I. K. Litvinov, A. O. Orlova, V. G. Maslov, A. V. Fedorov, A. Dubavik, F. Purcell-Milton, Y. K. Gun'ko and A. V. Baranov, Nanotechnology, 2016, 27, 075102 CrossRef CAS PubMed.
- Y. Li, Y. Zhou, H.-Y. Wang, S. Perrett, Y. Zhao, Z. Tang and G. Nie, Angew. Chem., Int. Ed., 2011, 50, 5860–5864 CrossRef CAS.
- B. Zhao, X. Gao, K. Pan and J. Deng, ACS Nano, 2021, 15, 7463–7471 CrossRef CAS PubMed.
- S. Liu, M. Kepenekian, S. Bodnar, S. Feldmann, M. W. Heindl, N. Fehn, J. Zerhoch, A. Shcherbakov, A. Pöthig, Y. Li, U. W. Paetzold, A. Kartouzian, I. D. Sharp, C. Katan, J. Even and F. Deschler, Sci. Adv., 2023, 9, eadh5083 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |