Sugar additive with a halogen group enabling a highly reversible and dendrite-free Zn anode†
Weihao
Xu
ab,
Xipo
Ma
ab,
Pengbo
Lyu
 c,
Zhenren
Gao
c,
Zhenren
Gao
 d,
Chunshuang
Yan
d,
Chunshuang
Yan
 *ab and
Chade
Lv
*ab and
Chade
Lv
 *ab
*ab
aState Key Laboratory of Space Power-Sources, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, P.R. China. E-mail: csyan@hit.edu.cn; lv.chade@hit.edu.cn
bMIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, P.R. China
cHunan Provincial Key Laboratory of Thin Film Materials and Devices, School of Material Sciences and Engineering, Xiangtan University, Xiangtan, 411105, P.R. China
dHunan Provincial Key Laboratory of Micro-nano Energy Materials and Application Technologies, College of Physics and Electronic Engineering, Hengyang Normal University, Hengyang 421002, P.R. China
First published on 29th October 2024
Abstract
Aqueous zinc-ion batteries (AZBs) suffer from poor reversibility and limited lifespan due to parasitic side reactions and dendrite growth induced by active water. Although additives are widely used to address these issues by reducing the water content within the Zn-ion solvation sheaths, the strong interaction between the additives and Zn2+ causes poor de-solvation kinetics. Here, we propose a strategy that introduces an electron-withdrawing halogen group to reduce the polarity of the sugar additive. Theoretical simulations and experimental results demonstrate that a sucralose additive with optimal polarity can decrease the coordinated active water without hindering the de-solvation kinetics of Zn2+. This effectively regulates the overpotential and inhibits side reactions. Meanwhile, the additive can adsorb onto the surface of the Zn metal to modify the direction of zinc deposition and suppress dendrite growth. As a result, the Zn//Zn symmetric cell with the sucralose electrolyte additive exhibits an outstanding cycling life of 2400 h at a current density of 1 mA cm−2. Moreover, when coupled with the V2O5 cathode, the full battery also demonstrates excellent operational stability, achieving 4000 cycles with a retained capacity of 51.84%.
1. Introduction
Aqueous zinc-ion batteries (AZBs) are considered a promising option for grid-scale energy storage systems and biocompatible devices due to their low cost and high safety.1–7 However, Zn metal anodes face several critical challenges, such as dendrite growth and side reactions in conventional aqueous electrolytes. These issues arise from the highly reactive water in the primary solvated shells of Zn2+.8–10 These H2O molecules are prone to the hydrogen evolution reaction (HER),11 leading to an increase in local OH− ion concentration and the formation of by-products at the electrolyte/Zn anode interface. Such continuous corrosion exacerbates the uneven electric field distribution, inducing inhomogeneous Zn2+ flux, which ultimately triggers Zn dendrite growth. These interfacial reactions contribute to low coulombic efficiency and poor cycling stability of the Zn anode, significantly hindering their commercialized applications.12,13To address these problems, various strategies have been proposed, including surface modification of the Zn anode,14 separator design,15,16 electrolyte optimization17,18 and current collector design.19 Among these, electrolyte engineering is regarded as a simple and effective method to mitigate side reactions and dendrite formation.20–24 So far, numerous electrolyte additives, such as dimethyl sulfoxide, N-methyl-2-pyrrolidone, xylitol, glucose, and sucrose, have been reported for their ability to regulate the solvation structure of Zn2+ and the electrolyte/anode interface,25–29 thereby reducing water reactivity, suppressing side reactions, and controlling Zn deposition. For example, sugars, as typical low-cost and non-toxic additives, have been explored in AZBs to modulate the solvation structures of Zn2+ and alleviate side reactions on Zn electrodes.30–32 However, due to the strong polarity of hydroxyl and carboxyl groups, the interaction between Zn2+ and these sugars may slow down the de-solvation kinetics of Zn2+, which is not conducive to the even diffusion of Zn2+ and may even aggravate the growth of Zn dendrites.30–32 Therefore, optimizing the group polarity of sugar is crucial for achieving good de-solvation kinetics of Zn2+ and obtaining a highly stable and dendrite-free Zn anode. Theoretically, the introduction of halogen groups with the ability to attract an electron will alter the overall group polarity of sugars. Additives with halogen groups have also been reported to build a hydrophobic environment to prevent corrosion of the Zn anode.33,34 Inspired by these studies, we speculate that sucralose with chlorine groups will exhibit superior performance compared with sucrose.
Herein, sucralose containing a halogen group, as a paradigm additive, is investigated by combining experimental measurements with theoretical simulations. Results show that sucralose additives can effectively restrain side reactions, inhibit dendrite growth and improve the cycling lifetime of the Zn anode. As a comparison, sucrose without a halogen group delivers inferior performance due to the strong interaction between sucrose and Zn2+, hindering the de-solvation process, whereas the interaction between sucralose and Zn2+ is optimal. As a result, the symmetric cell with 0.03 M sucralose electrolyte (SRL) additive presents an outstanding cycle performance at a current density of 1 mA cm−2 and capacity of 1 mA h cm−2 (2400 h), 5 mA cm−2 and capacity of 5 mA h cm−2 (700 h). Moreover, the Zn//V2O5 full cell displays excellent cycling stability (51.84% after 4000 cycles), which is better than that using the pure ZnSO4 electrolyte.
2. Experimental section
2.1. Electrolyte preparation
The 2 M ZnSO4 (ZSO) electrolyte was prepared by dissolving zinc sulfate (ZnSO4·7H2O, Sinopharm, AR) into deionized water. Sucralose (Aladdin, 98%) with different concentrations (0.01, 0.03, 0.05 M) was added to the as-obtained ZSO to form the experimental electrolytes. SRL specifically refers to the ZSO electrolyte containing 0.03 M sucralose. For comparison, the ZSO electrolyte containing 0.03 M sucrose was denoted as the SUC electrolyte.2.2. Electrode preparation
The zinc (Zn, thickness 100 μm) metal foils were used directly as the anode. The V2O5 cathode was prepared by mixing V2O5 powder (V2O5, Aladdin), carbon black and polyvinylidene fluoride (PVDF) with the mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidone (NMP, AR, >99.0%) solution. The obtained slurry was coated onto the titanium (Ti, thickness 0.1 mm) foil (about 1.2 mg cm−2). Then, the cathode was dried at 60 °C for 12 h in a vacuum oven. The mass loading of the cathode was about 1–2 mg cm−2.
1 in N-methyl-2-pyrrolidone (NMP, AR, >99.0%) solution. The obtained slurry was coated onto the titanium (Ti, thickness 0.1 mm) foil (about 1.2 mg cm−2). Then, the cathode was dried at 60 °C for 12 h in a vacuum oven. The mass loading of the cathode was about 1–2 mg cm−2.
2.3. Material characterization
The scanning electron microscope (SEM) images and energy-dispersive X-ray spectroscopy (EDS) were acquired using a field-emission scanning electron microscope (ZEISS SUPRA55). X-ray diffraction (XRD) patterns were recorded with X-ray diffractometer equipment (D8 ADVANCE, BRUKER). Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) measurements were conducted with the Nicolet Summit X. Raman spectroscopy measurements of electrolytes were collected by the RENISHAW PLUS with a laser wavelength of 532 nm. The contact angles of different electrolytes with the Zn anode were characterized by a contact angle measurement system (Shanghai Fangrui, JCY-1). In situ optical microscopy (DM2700M, Leica) and optical surface profilometry (ContourX-200, BRUKER) were performed to observe the flatness of the Zn anode surface.2.4. Electrochemical characterization
Electrochemical performances of the half and full batteries were evaluated using CR2032 coin cells with the Zn foil as the anode, glass fiber as the separator, and the as-prepared electrolyte as the electrolyte. The electrolyte dosage for each cell in our study was 100 μl. This specific amount was selected to optimize the electrochemical performance of the batteries.7,35 The Zn//Zn symmetric cells were assembled to test the reversibility of Zn plating/stripping. Galvanostatic charge/discharge (GCD) curves, rate performance and long-term cycling tests were recorded on a NEWARE battery-testing instrument (Shenzhen, China) at different current densities. The electrochemical impedance spectroscopy (EIS, from 100 kHz to 0.1 Hz), cyclic voltammetry (CV), linear scanning voltammetry (LSV) and chronoamperometry (CA) measurements were performed on an electrochemical workstation (AUTOLAB PGSTAT302N, Switzerland). LSV was performed using a three-electrode system with Zn foil as the working electrode, Pt plate as the counter electrode, Ag/AgCl as the reference electrode, and different zinc-based electrolytes as the electrolyte. The CA plots were measured with and without SRL using the above-mentioned three-electrode system at an overpotential of −150 mV and a duration of 600 s. According to a previous report,36 the electrical double-layer capacitance (EDLC) was calculated by the equation of Cdl = i/v (Cdl: capacitance, i: current, v: corresponding sweep speed. Among them, i was defined by half of the difference between the positive and negative scanning current at each scanning rate).37,38 The full cells were tested in the voltage range of 0.4–1.6 V.2.5. Density functional theory (DFT) calculations
The structural optimization and simulation calculation of simple small molecules were carried out by Gaussian 16 software39 based on density functional theory (DFT) at room temperature and pressure. The method uses DFT B3LYP-GD3BJ, and the basis set is 6-311G(d,p) to simulate the simple small molecule model. For the calculation of the binding energy, we have considered the basis set superposition error (BSSE). GaussView 6.0.16 (ref. 40) was used for visualization of the results. In addition, first-principles calculations were performed based on DFT and the projector augmented wave (PAW) potential.41 They were implemented in the Vienna Ab initio Simulation Package (VASP).42 Exchange–correlation energy was treated using the generalized gradient approximation (GGA) parameterized by the Perdew–Burke–Ernzerhof (PBE)43 functional. Wave functions were expanded in a plane-wave basis of 500 eV kinetic energy cut-off. Atomic positions were fully relaxed with a force convergence criterion of 0.05 eV Å−1 and an electron self-consistent convergence criterion of 1 × 10−4 eV. The DFT-D3 method with Becke–Johnson damping was considered.3. Results and discussion
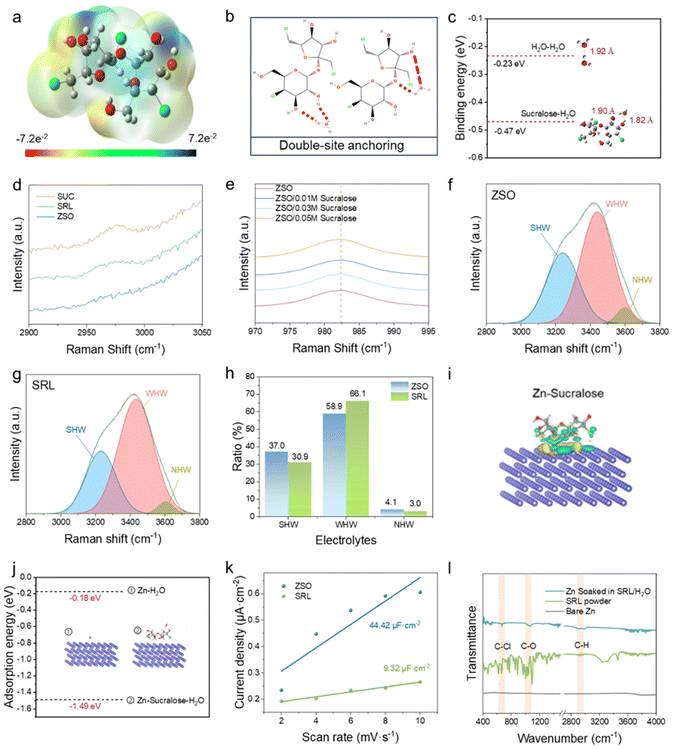
The structure of the sucralose molecule is similar to that of the sucrose molecule (Fig. S1†), and it corresponds to the replacement of the three hydroxyl groups in sucrose by chlorine atoms. Density functional theory (DFT) calculations were applied to obtain the electrostatic potential of a sucralose molecule (Fig. 1a). As can be seen from Fig. 1a, the electrostatic potential distribution is not homogeneous for the sucralose molecule, which has less polarity than sucrose.44 This might keep the sucralose molecules from strongly binding with H2O (Fig. 1b) and Zn2+, and facilitates the Zn2+ de-solvation kinetics. DFT calculations proved that sucralose molecules enabled two binding forms with H2O molecules, of which the binding energies were higher than that between H2O molecules (Fig. 1c). This suggests that sucralose molecules can more readily bind to H2O molecules, thereby disrupting the hydrogen bonding network of the pristine ZSO electrolyte. By analysis of the Raman spectra (Fig. 1d), the sucrose–H2O and sucralose–H2O hydrogen bonding were determined in the SUC and SRL electrolytes. In contrast, the absence of such bonds in the ZSO electrolyte demonstrates the formation of the hydrogen bonds with H2O for both sucralose molecules and sucrose molecules. Therefore, the sucrose molecules with higher polarity are more hydrophilic. According to the Raman spectra (Fig. 1e), the peaks of SO42− were not displaced or shielded by the signals in the electrolytes with different sucralose amounts. This indicates that the electrostatic interaction between Zn2+ and SO42− does not significantly change. No strong interaction between SO42− and sucralose further suggests that the promotion in the de-solvation kinetics of Zn2+ is attributed to the addition of sucralose. The Raman spectra of the ZSO electrolyte and SRL electrolytes were further fitted at the H2O band (Fig. 1f and g). In these two spectra, H2O molecules exist in three forms, including the strong hydrogen-bonded water (SHW) mainly in the form of four H-bonds, non-hydrogen-bonded water (NHW), and weakly hydrogen-bonded water (WHW).8,45,46Obviously, the addition of the sucralose additive attenuates the hydrogen bonding network of the pristine ZSO electrolyte (Fig. 1h). Subsequently, we performed Fourier-transform infrared (FTIR) analysis to investigate the changes in the solvation structure (as shown in Fig. S3†). The introduction of sucralose led to a blue shift in the O–H stretching vibrational band (3000–3700 cm−1) and the O–H bending vibrational band (1500–1700 cm−1), suggesting a reduction in the hydrogen bonding between the water molecules. Concurrently, the red shift observed in the SO42− vibrational band signifies a weakened interaction between Zn2+ and SO42−. These findings confirm that sucralose molecules are capable of diminishing the hydration solvation of Zn2+ and disrupting the hydrogen-bonded networks.
Moreover, the proportion of SHW in the SRL electrolyte decreased, while the WHW increased. This further affirmed the results obtained from the DFT calculations. DFT calculations further demonstrated that there was significant charge transfer between the Zn anode and the sucralose molecules. Such strong chemisorption forms an adsorption layer with abundant sucralose molecules (Fig. 1i). As a result, H2O molecules would be confined outside the sucralose adsorption layer, which could also control the H2O content on the Zn anode surface to form the H2O-poor electric double-layer (EDL). The adsorption energy of Zn–sucralose–H2O is much larger than that of Zn–H2O (Fig. 1j). The sucralose molecules preferentially adsorb on the surface of the Zn anode relative to H2O, which induces the shielding effect on the H2O molecules by forming a H2O-poor electric double-layer (EDL). The results show that the EDLC of the Zn electrode decreases in the SRL electrolyte due to the adsorption of sucralose molecules, which causes insufficient active sites (Fig. 1k and S2†). ATR-FTIR recorded on Zn foils soaked in SRL electrolyte confirmed the adsorption of sucralose molecules onto the Zn foils, as reflected by the presence of the C–Cl functional groups (Fig. 1l). EDS mappings on the cycled Zn anodes clearly demonstrated that the elemental densities of O, Cl, and C were much higher on the deposition surface (top) than that on the cross-section (bottom) (Fig. S4†). This further validated the adsorption of sucralose molecules on the Zn anode.
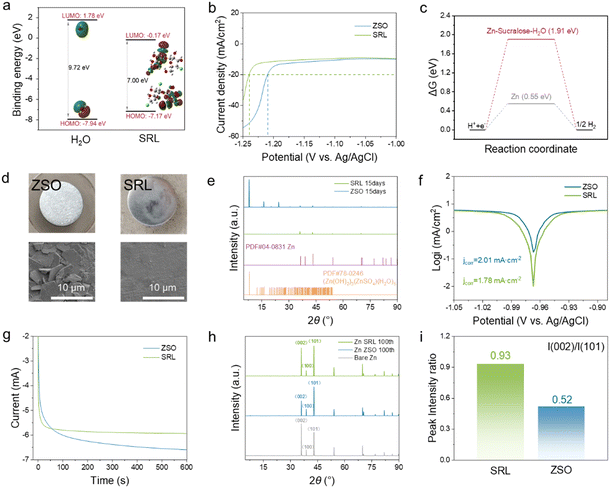
The adsorption of sucralose molecules on the Zn anode reduces the occurrence of HER on its surface and the corresponding by-products.11 By calculating the energy levels of the molecular orbitals, it is clear that the energy gap between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of the sucralose molecules is much smaller than that of H2O (7.00 eV vs. 9.72 eV), suggesting much stronger transfer of electrons after absorbing sucralose molecules on the surface of the Zn anode (Fig. 2a).
As shown in Fig. 2b and S5,† the SRL electrolyte possessed greater water splitting overpotentials, which agreed with the higher hydrogen precipitation reaction energy barrier based on the DFT calculations results (Fig. 2c). Optical photography, SEM and XRD were conducted to analyse the stability of the Zn foils soaked in different electrolytes for 15 days (Fig. 2d and e). The surface of the Zn foil soaked in the ZSO electrolyte was relatively rougher than that of the Zn foil immersed in the SRL electrolyte because of the generation of by-products. This suggests that the SRL electrolyte inhibits the occurrence of side reactions on the Zn surface, and significantly attenuates hydrogen precipitation and by-product generation. The XRD results suggested the peaks from the Zn(OH2)2(ZnSO4)(H2O)5 by-product for Zn soaked in the ZSO electrolyte, as compared to the SRL electrolyte. Tafel curves measured using the three-electrode system show that a significantly smaller corrosion current was detected for the SRL electrolyte, which also proved that the use of sucralose reduced the corrosion current density at the Zn electrode, demonstrating the superior ability of the sucralose additive to inhibit the Zn anode self-corrosion (Fig. 2f). Chronoamperometry (CA) tests were also carried out on different electrolytes for Zn//Zn symmetric cells at a constant overpotential of −150 mV. In the SRL electrolyte, the current density in the CA curve briefly decreased at the beginning of the deposition and then levelled off. In contrast, the CA curve for the ZSO electrolyte showed a continuous increase over 600 s (Fig. 2g). In the ZSO electrolyte, it followed a two-dimensional (2D) diffusion mechanism for Zn deposition over a longer period of time. During this planar diffusion process, Zn2+ diffused laterally along the deposited surface and accumulated at the top of the deposited Zn, eventually leading to the growth of Zn dendrites. In the SRL electrolyte, however, transient two-dimensional diffusion was followed by a continuous three-dimensional (3D) diffusion of the Zn deposition. Thus, during this process, sucralose selectively adsorbs on the nucleation sites, limiting the Zn2+ diffusion and impeding the 2D diffusion for directionally dense deposition of Zn2+.41
Furthermore, we employed the COMSOL simulation to demonstrate the role of the sucralose electrolyte additive in controlling the interfacial flow of Zn ions, as shown in ESI,† Fig. S6. The scratches and bumps, which are inevitable during processing, would enhance the local field strength, resulting in non-uniform Zn deposition and the growth of dendrites due to the “tip effect”. The simulation results indicate that the Zn-ion flow is indeed more focused on the zinc tip, causing the continuous dendrite growth with the consumption of the Zn electrode and electrolyte. With the extension of the deposition time, significant damage and uncontrolled dendrite formation would be visible on the electrode surface. However, by adding the sucralose electrolyte additive, the distribution of the Zn2+ flux in this configuration is effectively evened out, with no apparent accumulation of the tip deposition. The entire electrode surface becomes smooth, with uniform and dense Zn deposition, and the gaps between the particles are progressively filled as the deposition time is extended. Subsequently, the Zn2+ values were determined by conducting chronoamperometric (CA) tests on the Zn symmetric cell and analyzing the electrochemical impedance spectroscopy (EIS) data at both initial and steady states, as depicted in Fig. S7.† Based on the CA profiles and EIS data from the two electrolyte setups, and utilizing the Zn2+ calculation method outlined in the ESI,† it was inferred that the Zn2+ value for the cell containing the additive was higher compared to the cell without the additive. This enhancement is attributed to the polar hydroxyl groups of the sucralose additive, which interact strongly with Zn2+, leading to a uniform Zn2+ flow and a reduction in the interfacial concentration gradient by promoting the movement of Zn2+. In the interaction with Zn2+, both sucrose and sucralose molecules exhibit strong binding abilities, with binding energies of −11.56 eV and −9.35 eV, respectively.44 The relatively weaker binding energy of sucralose with Zn2+ is a direct reflection of its reduced polarity. This weak interaction with zinc ions is beneficial for the de-solvation process of Zn2+.
According to the XRD patterns (Fig. 2h), the Zn2+ electrodeposition in the ZSO electrolyte was more inclined to be deposited on the (101) crystal plane. Conversely, electrodeposition was more likely to occur on the (002) crystal plane for the SRL electrolyte. The (002) diffraction intensity in the SRL electrolyte was significantly higher than that in the ZSO electrolyte for the same type of Zn foils cycled for 100 h. The (002) diffraction intensity in the SRL electrolyte was significantly higher than that in the ZSO electrolyte. The (002)/(101) diffraction intensity ratio for the Zn foil after cycling in the SRL electrolyte was higher than that of the Zn foil in the ZSO electrolyte (0.93 vs. 0.52) (Fig. 2i).
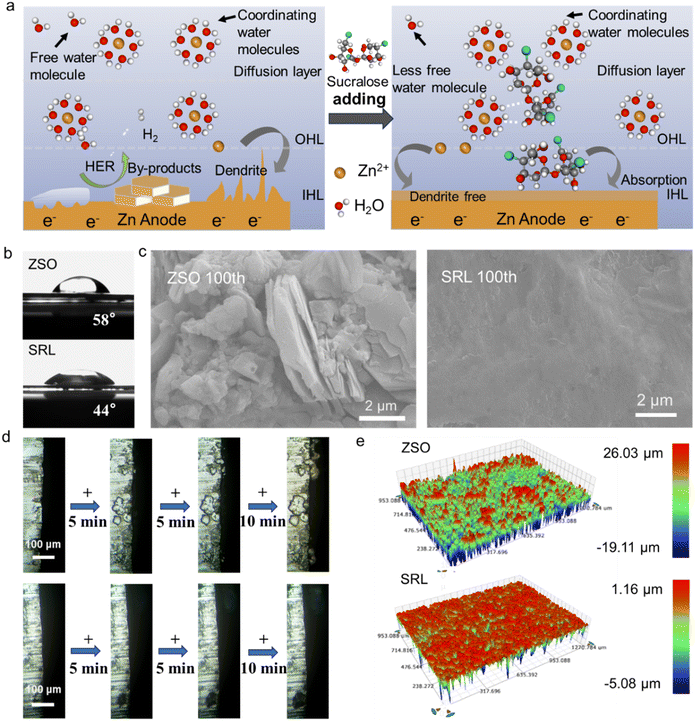
Fig. 3a shows the electrodeposition of Zn2+ in ZSO and the SRL electrolytes in detail. The H2O molecules were more susceptible to side reactions on the Zn anode surface in the ZSO electrolytes, which resulted in the production of H2 and other by-products during the long cycling test. Therefore, the haphazard Zn2+ electrodeposition might occur on the rough surface of the Zn anode after the side reactions, leading to the generation of Zn dendrites to potentially puncture the separator. Conversely, owing to the formation of EDL on the surface of the Zn anode during the electrochemical deposition of Zn2+ in the SRL electrolyte, H2O molecules were impeded from inducing the orderly deposition of Zn2+ on the (002) crystalline surfaces, resulting in highly reversible and dendrite-free Zn anode.
The wettability of the electrolyte was quantified by measuring the contact angle. The SRL electrolyte has a smaller contact angle with the Zn anode compared to the ZSO electrolyte (Fig. 3b). This result suggested that the electrolyte was more Zn-friendly in the presence of the sucralose additive. The Zn//Zn symmetric cell was cycled for 100 h in different electrolytes (at a current density of 1 mA cm−2 and capacity of 1 mA h cm−2). Observation of the Zn foils after cycling by SEM (Fig. 3c) showed that the dendrites appeared on the surface of the Zn foils cycled in the ZSO electrolyte, whereas the Zn foils in the SRL electrolyte were significantly flatter without obvious Zn dendrite generation. The optical photographs of the same Zn anode foil also demonstrated that Zn2+ was deposited more uniformly in the SRL electrolyte (Fig. S8†).
In the in situ optical microscopy observations (Fig. 3d), pronounced dendrite growth on Zn foils in the ZSO electrolyte was detected. However, in the SRL electrolyte, the growth of zinc dendrites (indicated by the black shadow below) is significantly inhibited. Uniform Zn2+ deposition was realized in the SRL electrolyte and no significant dendrite growth was also observed, as reflected in the optical profiling as well (Fig. 3e). In sharp contrast, the surface of the Zn foil in the ZSO electrolyte showed significant dendrite generation, and with an order of magnitude higher in the surface elevation difference.
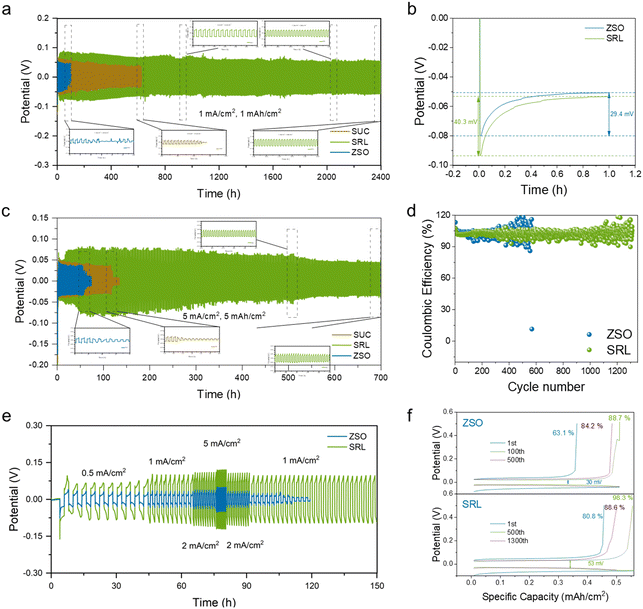
To affirm the significant role of the sucralose additive, the Zn//Zn symmetric cells were tested under different conditions.44 Firstly, the Zn//Zn symmetric cells were tested with different sucralose concentrations added in the electrolytes. With 0.03 M sucralose, the Zn//Zn symmetric cell enabled the longest cycle life (Fig. S9†). By comparison with the ZSO electrolyte, the symmetric cell in the SRL electrolyte presented outstanding cycling performance at the current density of 1 mA cm−2 and the capacity of 1 mA h cm−2 (2400 h vs. 100 h), 5 mA cm−2 and the capacity of 5 mA h cm−2 (700 h vs. 80 h) (Fig. 4a and b). This highlighted the advantage of the SRL electrolyte with the sucralose additive.
The SRL electrolyte could also improve the interfacial transport kinetics in the Zn//Zn symmetric cells, which was reflected in the higher rate performance compared to that in the ZSO electrolyte (Fig. 4c and S10†). Likewise, the electrolyte cell containing sucralose also exhibited superior rate performance compared to that with SUC electrolyte (Fig. S11†). Zn//Cu asymmetric cells were further prepared to analyze the nucleation overpotential of Zn2+.47–51 The SRL electrolyte displayed a higher nucleation overpotential (40.3 mV) in contrast with those in ZSO (29.4 mV) and SUC (34.8 mV) electrolytes (Fig. 4d and S12†). This indicated that the Zn2+ nucleation in the SRL electrolyte was smaller and denser on the surface of the Cu foil, which was conducive to improving the cycling stability (Fig. S13†).
In addition, Fig. 4e and f illustrates the better electrochemical stability of the Zn//Cu asymmetric cell with the SRL electrolyte relative to that with the ZSO electrolyte. In the ZSO electrolyte, the average coulombic efficiency (CE) of the Zn//Cu cells was relatively low (96.85%) and unstable with a short cycling life (1 mA cm−2, 0.5 mA h cm−2). In contrast, the average CE was more stable and higher (99.02%) in the SRL electrolyte, which endowed the Zn//Cu cells with a considerably increased cycling life of >1200 cycles. The corresponding voltage distributions of the cells using the SRL electrolyte were smoother and more stable at the 1st, 500th and 1300th cycles. In contrast, the voltage distribution curves of the cells using the ZSO electrolyte showed a short circuit after 550 cycles, and the corresponding polarisation voltages were unstable during the charging and discharging processes.
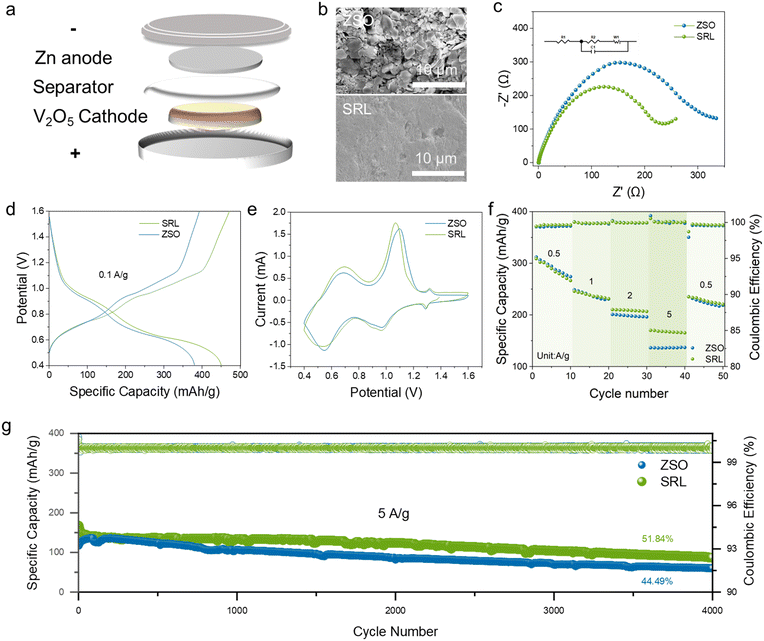
To evaluate the usefulness of the sucralose additives in practical battery systems, Zn//V2O5 full cells20,30,52–55 were assembled using commercial V2O5 powders as the cathode active material (Fig. 5a). All the Zn//V2O5 full cells were measured after the activation of the V2O5 cathode material, of which the morphology is illustrated in Fig. S14.† SEM characterizations were conducted on the Zn anodes of the Zn//V2O5 full cells cycled at the current density of 5 A g−1 for 4000 cycles. Obvious Zn dendrites could be observed on the Zn anode in the ZSO electrolyte, while the surface of the Zn anode cycled in the SRL electrolyte was much flatter (Fig. 5b). Based on this result, the Zn//V2O5 full cell would deliver a longer cycle life in the SRL electrolyte. In addition, the electrochemical impedance spectra (EIS) plots of the Zn//V2O5 full cells indicated lower interfacial impedance in the SRL electrolyte (Fig. 5c), manifesting the good compatibility of the interfacial protective layer.56,57 The CV analysis recorded on the Zn//V2O5 cells exhibited two CV curves with similar redox peaks, implying that the electrochemical processes were reversible with the SRL electrolyte (Fig. 5d). At the current density of 0.1 A g−1, the Zn//V2O5 full cell delivered a higher specific capacity (450 mA h g−1) with the SRL electrolyte than that with the ZSO electrolyte (380 mA h g−1). The rate performance of the Zn//V2O5 full cell in the presence of sucralose was also superior to that of the ZSO electrolyte (Fig. 5f). Fig. 5g and S15† display the long cycling performance at a current density of 5 A g−1. After approximately 4000 cycles, the capacity retention rates of the Zn//V2O5 full cells with ZSO and SUC electrolytes were 44.49% and 51.78%, respectively. In contrast, the SRL electrolyte endowed the Zn//V2O5 full cell with higher stability in terms of its 51.84% capacity retention rate. Meanwhile, the average CE increased significantly from 99.91% to 99.97% with the addition of sucralose. Fig. S16† displays the long cycling performance at a current density of 2 A g−1. After approximately 1000 cycles, the capacity retention rates of the Zn//V2O5 full cells with ZSO and SRL electrolytes were 26.46% and 46.95%, respectively. This indicates that the use of sucralose as an electrolyte additive also enhances the cycling performance of the full cell at low current densities.
4. Conclusions
In summary, we have reported the use of sucralose as an electrolyte additive in the AZBS. The experimental and DFT calculation results show that sucralose with a halogen group can reduce the polarity of the hydroxyl group to boost the de-solvation kinetics of Zn2+. It also can combine with H2O molecules and destroy the original hydrogen bonding network of the electrolyte, thereby reducing the activity of H2O. Sucralose can preferentially adsorb on the surface of the Zn anode, which forms a H2O-poor electric double-layer (EDL) and reduces the occurrence of HER, enabling highly reversible and dendrite-free Zn anodes. The adsorption of sucralose on the surface of the Zn anode also enables the deposition of Zn2+ to achieve the crystalline surface orientation. As a result, the electrochemical performance of the Zn//Zn symmetric cell and Zn//V2O5 full cell using the SRL electrolyte additive is better than that of the battery using SUC or pure ZSO electrolytes. These insights contribute to the design of more efficient and reliable electrolyte additives for diverse energy storage devices.Data availability
The data will be made available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. L. acknowledges the National Natural Science Foundation of China (52302231 and 22479034). C. Y. acknowledges the National Natural Science Foundation of China (52101246), and the Natural Science Foundation of Heilongjiang Province, China (YQ2022B006). P. L. acknowledges the Hunan Provincial Natural Science Foundation of China (2023JJ40621). The authors would like to thank SCI-GO (https://www.sci-go.com) for the XPS analysis.References
- E. Hu and X. Yang, Nat. Mater., 2018, 17, 480–481 CrossRef CAS.
- M. Rana, N. Alghamdi, X. Peng, Y. Huang, B. Wang, L. Wang, I. R. Gentle, S. Hickey and B. Luo, Exploration, 2023, 3, 20220073 CrossRef CAS PubMed.
- X. Zheng, R. Luo and W. Chen, Nano Res. Energy, 2023, 2, 9120053 CrossRef.
- S. Wu, Z. Hu, P. He, L. Ren, J. Huang and J. Luo, eScience, 2023, 3, 100120 CrossRef.
- S. Bai, Z. Huang, G. Liang, R. Yang, D. Liu, W. Wen, X. Jin, C. Zhi and X. Wang, Adv. Sci., 2024, 11, 2304549 CrossRef CAS.
- J. Li, Z. Liu, S. Han, P. Zhou, B. Lu, J. Zhou, Z. Zeng, Z. Chen and J. Zhou, Nano-Micro Lett., 2023, 15, 237 CrossRef CAS.
- H. Ge, L. Qin, B. Zhang, L. Jiang, Y. Tang, B. Lu, S. Tian and J. Zhou, Nanoscale Horiz., 2024, 9, 1514–1521 RSC.
- T. Sun, H. Du, S. Zheng, J. Shi and Z. Tao, Adv. Funct. Mater., 2021, 31, 2010127 CrossRef CAS.
- F. Jing, C. Lv, L. Xu, Y. Shang, J. Pei, P. Song, Y. Wang, G. Chen and C. Yan, J. Energy Chem., 2023, 87, 314–321 CrossRef CAS.
- Z. Chen, Q. Wu, X. Han, C. Wang, J. Chen, T. Hu, Q. He, X. Zhu, D. Yuan, J. Chen, Y. Zhang, L. Yang, Y. Ma and J. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202401507 CrossRef CAS.
- G. Wu, Y. Yang, R. Zhu, W. Yang, H. Yang and H. Zhou, Energy Environ. Sci., 2023, 16, 432–4325 Search PubMed.
- Z. Miao, Q. Liu, W. Wei, X. Zhao, M. Du, H. Li, F. Zhang, M. Hao, Z. Cui, Y. Sang, X. Wang, H. Liu and S. Wang, Nano Energy, 2022, 97, 107145 CrossRef CAS.
- Z. Yi, G. Chen, F. Hou, L. Wang and J. Liang, Adv. Energy Mater., 2021, 11, 2003065 CrossRef CAS.
- J. Cao, H. Wu, D. Zhang, D. Luo, L. Zhang, X. Yang, J. Qin and G. He, Angew. Chem., Int. Ed., 2024, 63, e202319661 CrossRef CAS.
- J. Abdulla, J. Cao, P. Wangyao and J. Qin, J. Met., Mater. Miner., 2020, 30, 1–8 CrossRef CAS.
- C. Yang, P. Woottapanit, Y. Yue, S. Geng, J. Cao, X. Zhang, G. He and J. Qin, Small, 2024, 20, 2311203 CrossRef CAS PubMed.
- J. Cao, Y. Sun, D. Zhang, D. Luo, H. Wu, X. Wang, C. Yang, L. Zhang, X. Yang and J. Qin, ChemElectroChem, 2024, 11, e202400064 CrossRef CAS.
- J. Cao, X. Wang, D. Zhang, R. Chanajaree, L. Zhang, J. Qin and X. Yang, J. Alloys Compd., 2024, 972, 172773 CrossRef CAS.
- Z. Li, T. T. Beyene, K. Zhu and D. Cao, J. Met., Mater. Miner., 2024, 34, 2009 CrossRef CAS.
- W. Du, E. H. Ang, Y. Yang, Y. Zhang, M. Ye and C. C. Li, Energy Environ. Sci., 2020, 13, 333–336 RSC.
- K. Lu, B. Song, Y. Zhang, H. Ma and J. Zhang, J. Mater. Chem. A, 2017, 5, 23628–23633 RSC.
- T. Xiong, Y. Zhang, Y. Wang, S. V. L. Wee and J. Xue, J. Mater. Chem. A, 2020, 8, 9006–9012 RSC.
- B. Wu, Y. Wu, Z. Lu, J. Zhang, N. Han, Y. Wang, X. Li, M. Lin and L. Zeng, J. Mater. Chem. A, 2021, 9, 4734–4743 RSC.
- J. Yin, Y. Wang, Y. Zhu, J. Jin, C. Chen, Y. Yuan, Z. Bayhan, N. Salah, N. A. Alhebshi, W. Zhang, U. Schwingenschlögl and H. N. Alshareef, Nano Energy, 2022, 99, 107331 CrossRef CAS.
- W. Lv, J. Meng, X. Li, C. Xu, W. Yang, S. Duan, Y. Li, X. Ju, R. Yuan, Y. Tian, M. Wang, X. Lyu, P. Pan, X. Ma, Y. Cong and Y. Wu, Energy Storage Mater., 2023, 54, 784–793 CrossRef.
- S. Li, Y. Xu, X. Xu, L. Shi, L. Sun, H. Wan, W. Zhuang and M. Song, J. Electron. Mater., 2024, 53, 408–417 CrossRef CAS.
- M. Song and C. L. Zhong, Rare Met., 2022, 41, 356–360 CrossRef CAS.
- H. Dou, X. Wu, M. Xu, R. Feng, Q. Ma, D. Luo, K. Zong, X. Wang and Z. Chen, Angew. Chem., Int. Ed., 2024, 63, e202401974 CrossRef CAS.
- W. Kao-ian, J. Sangsawang, P. Kidkhunthod, S. Wannapaiboon, M. Suttipong, A. Khampunbut, P. Pattananuwat, M. T. Nguyen, T. Yonezawa and S. Kheawhom, J. Mater. Chem. A, 2023, 11, 10584–10595 RSC.
- C. Wang, J. Hou, Y. Gan, L. Xie, Y. He, Q. Hu, S. Liu and S. Chan Jun, J. Mater. Chem. A, 2023, 11, 857–865 Search PubMed.
- F. Jing, Y. Liu, Y. Shang, C. Lv, L. Xu, J. Pei, J. Liu, G. Chen and C. Yan, Energy Storage Mater., 2022, 49, 164–171 CrossRef.
- X. Ma, H. Yu, C. Yan, Q. Chen, Z. Wang, Y. Chen, G. Chen and C. Lv, J. Colloid Interface Sci., 2024, 664, 539–548 CrossRef CAS PubMed.
- S. Xin, J. Xie, J. Wang, S. Xie, Z. Yang and X. Lu, Nat. Commun., 2024, 15, 302 CrossRef.
- H. Wang, A. Zhou and X. Hu, ACS Nano, 2023, 12, 11946–11956 CrossRef.
- X. Yi, H. Fu, A. M. Rao, Y. Zhang, J. Zhou, C. Wang and B. Lu, Nat. Sustain., 2024, 7, 326–337 CrossRef.
- C. Wu, C. Sun, K. Ren, F. Yang, Y. Du, X. Gu, Q. Wang and C. Lai, Chem. Eng. J., 2023, 452, 139465 CrossRef CAS.
- J. Chen, Z. Jin, W. Dou and J. Subotnik, J. Phys. Chem. C, 2021, 125, 2884–2899 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- M. Frisch, et al., Gaussian 16, Revision A. 03, Gaussian, Inc, Wallingford, 2016 Search PubMed.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView 6.0. 16, Semichem Inc., Shawnee Mission, KS, USA, 2016, pp. 143–150 Search PubMed.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- Q. He, T. Hu, Q. Wu, C. Wang, X. Han, Z. Chen, Y. Zhu, J. Chen, Y. Zhang, L. Shi, X. Wang, Y. Ma and J. Zhao, Adv. Mater., 2024, 36, 2400888 CrossRef CAS PubMed.
- Q. Zhang, Y. Ma, Y. Lu, L. Li, F. Wan, K. Zhang and J. Chen, Nat. Commun., 2020, 11, 4463 CrossRef CAS PubMed.
- K. Su, X. Zhang, X. Zhang, C. Wang, Y. Pu, Y. Wang, S. Wan and J. Lang, Chem. Eng. J., 2023, 474, 145730 CrossRef CAS.
- R. Yao, Y. Zhao, L. Wang, C. Xiao, F. Kang, C. Zhi and C. Yang, Energy Environ. Sci., 2024, 17, 3112–3122 RSC.
- L. Cao, D. Li, T. Pollard, T. Deng, B. Zhang, C. Yang, L. Chen, J. Vatamanu, E. Hu, M. J. Hourwitz, L. Ma, M. Ding, Q. Li, S. Hou, K. Gaskell, J. T. Fourkas, X. Q. Yang, K. Xu, O. Borodin and C. Wang, Nat. Nanotechnol., 2021, 16, 902–910 CrossRef CAS PubMed.
- S. Chen, D. Ji, Q. Chen, J. Ma, S. Hou and J. Zhang, Nat. Commun., 2023, 14, 3526 CrossRef CAS.
- D. Yuan, X. Li, H. Yao, Y. Li, X. Zhu, J. Zhao, H. Zhang, Y. Zhang, E. T. J. Jie, Y. Cai and M. Srinivasan, Adv. Sci., 2023, 10, 2206469 CrossRef CAS.
- X. Guo and G. He, J. Mater. Chem. A, 2023, 11, 11121–11987 Search PubMed.
- D. Chen, X. Rui, Q. Zhang, H. Geng, L. Gan, W. Zhang, C. Li, S. Huang and Y. Yu, Nano Energy, 2019, 60, 171–178 CrossRef CAS.
- Y. Mei, Y. Liu, W. Xu, M. Zhang, Y. Dong and J. Qiu, Chem. Eng. J., 2023, 452, 139574 CrossRef CAS.
- J. Sun, J. Zhang, S. Wang, P. Sun, J. Chen, Y. Du, S. Wang, I. Saadoune, Y. Wang and Y. Wei, Energy Environ. Sci., 2024, 17, 434–4318 Search PubMed.
- J. Song, L. Kou, Y. Wang, Y. Pang, T. Ai, K. Kajiyoshi, M. Liu, W. Bao, W. Li and P. Wattanapaphawong, React. Chem. Eng., 2023, 8, 1545–1552 RSC.
- Y. Li, Y. Li, Q. Liu, Y. Liu, T. Wang, M. Cui, Y. Ding, H. Li and G. Yu, Angew. Chem., Int. Ed., 2024, 63, e202318444 CrossRef CAS PubMed.
- Y. Geng, H. Fu, Y. Hu, A. M. Rao, L. Fan, J. Zhou and B. Lu, Appl. Phys. Lett., 2024, 124, 063901 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4re00366g |
| This journal is © The Royal Society of Chemistry 2025 |