pH Sensing with mesoporous thin films
Gernot Wirnsberger*a, Brian J. Scottb and Galen D. Stucky*b
aInorganic Chemistry, Karl-Franzens-University Graz, 8010, Graz, Austria.. E-mail: gernot.wirnsberger@kfunigraz.ac.at
bDepartment of Chemistry, University of California, Santa Barbara, CA- 93106, Santa Barbara, USA.. E-mail: stucky@chem.ucsb.edu
First published on 18th December 2000
Abstract
Optically clear thin mesoporous films with covalently attached fluorescein entities are shown to exhibit very fast response pH sensing.
Dye-doped mesostructured compounds are currently attracting interest with respect to possible optical applications such as materials exhibiting amplified spontaneous emission and lasing.1–3 The mesoporous forms are also promising for optical applications when doped with dyes. Dyes can be attached covalently to the matrix by introducing hydrolysable units onto the dye molecules.4 Principally this should allow one to remove the surfactants quantitatively, leaving behind a porous matrix with anchored dye molecules. The porous structure is especially important for optical sensing applications where fast diffusion of the molecules towards the sensing species is of interest.
Previous investigations on optical sensors has focused predominately on sol–gel glasses. The attractive points of sol–gel glasses are their ease of processibility as well as thermal and mechanical robustness. A few examples include oxygen sensing by Ru-complexes,5 pH sensing,6 and ion sensing.7 As far as pH sensing is concerned, the diffusion of the molecules into the dried glass is sometimes a limiting factor, usually leading to rather long response times (up to minutes),7 a task which could be improved with mesoporous materials. Here, we show that mesosporous thin films8 prepared by acidic sol–gel chemistry and low-temperature block-copolymer extraction9 can function very effectively as pH sensors. The pH sensitive dye is covalently anchored to/within the mesoporous SiO2 wall in order to prevent leaching during optical pH measurements. We also demonstrate that the response of the dye-modified mesoporous silica thin films is very fast, indicating the possibility of using these materials in sensor devices.
Fluorescein isothiocyanate (FITC) was used as the pH sensitive dye and was derivatized with 3-aminopropyltriethoxysilane (APTS). Typically, 7 mg of FITC was dissolved in 20.4 g of EtOH and was allowed to react with 10 mg APTS for 3 h. Afterwards, 2.0 g of the block-copolymer F127 [poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide), (PEO)106(PPO)70(PEO)106, BASF], 1.6 g H2O, 0.25 g HCl (2 M) and 8.5 g tetraethylorthosilicate (TEOS) were added. This solution with a molar composition of TEOS–F127–H2O–HCl–FTIC–APTS –EtOH = 1∶4 × 10−3∶2.5∶0.012∶4.4 × 10−4∶1.1 × 10−3∶10.9 was refluxed for 1 h. After the solution had cooled to rt, thin films were prepared by dip coating. Films were withdrawn from solutions with 10 cm min−1, resulting in ∼900 nm thick films on an area of 2.5 × 2.5 cm2. The films show a considerable thickness variation when prepared by dip-coating. Apart from the fact that the ends are swelled thick, the film thickness increases along the dipping direction. When the same solutions are spin-coated (3000 rpm, 30 s), more homogenous thin films (∼1500 nm) are produced, with a thickness variation of 5% (apart from the edges which are also swelled thick).
A typical X-ray pattern of an as-synthesized thin film is depicted in Fig. 1(a), showing several peaks in the region between 0.8 and 3.5 2θ°. The peaks can be indexed in the P63/mmc space group indicating that the composite thin film consists of a hexagonally packed arrangement of cages (a = 113, c = 181 Å, c/a = 1.60).8 One further non-indexable peak is observed at a d spacing of 51 Å; this peak may be attributed to the 200 reflection of a cubic Im3m phase which can also be derived under similar conditions with F127 as a block-copolymer.8 The 110 reflection is hidden by the intense reflection of the hexagonal structure at ∼1° 2θ. From the relative intensities we conclude that the hexagonal phase is the major part, whereas the cubic phase is only present in small amounts. However, as both structures are composed of cage-like entities, and porosity itself determines the sensing properties, the presence of minor amounts of a second phase is not of significant importance. We note also that the use of higher concentrations of APTS did not result in ordered mesostructures. This fact is attributed to the pH increase brought about by the basic amine. Hence, we adjusted the concentration of APTS carefully, in order to have an access for complete dye derivatization but still having a pH resulting in thin films with mesostructural order.
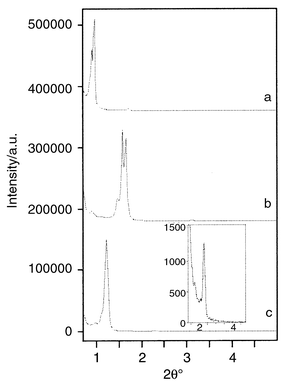 | ||
| Fig. 1 X-Ray diffraction patterns of fluorescein-doped thin mesostructured/mesoporous films prepared with F127. (a) As-synthesized, (b) after calcination, and (c) after extraction. The inset in (c) shows a magnification of the higher 2θ° range. | ||
A key point is the low temperature removal of the surfactants. Fresh films (∼1 d old) show severe cracking when treated in EtOH at 80 °C. The same is true, even when the films are aged for several days at rt. However, we observed that when the films had first been aged at rt for about 10 d and afterwards for 3–5 d at 70 °C, the stability of these films is sufficiently high to allow surfactant removal by extraction without decrease of the optical transparency.
The extraction time itself was optimized in order to completely remove the surfactants. IR spectroscopy was used to follow the surfactant removal during extraction by monitoring the C–H vibrations in the range 2700–3000 cm−1. Within the first 4 h of extraction, the intensity of these bands steadily decreased, until finally only weak bands in this wavelength region remained. These bands were still present after 24 h of extraction and may be attributed to vibrations due to the occluded dye and the excess APTS used for the dye derivatization. X-Ray patterns showed that prolonged treatment in EtOH at 80 °C results in a decrease of the unit-cell constants compared to the as-synthesized material (Fig. 1c). The unit-cell parameters (a = 90.1, c = 144.6 Å, c/a = 1.60) are larger than those of a calcined thin film made from the same composition (a = 69.8, c = 111.8 Å, c/a = 1.62), showing a lower contraction of the SiO2 framework when employing extraction for block-copolymer removal. We note that even after prolonged treatment in EtOH (24 h) not only the optical transparency and the mesostructure are retained but also the X-ray patterns exhibit all reflections seen for the as-synthesized material.
For the demonstration of pH sensing, we chose a simple configuration in which the thin films were excited by the 488 nm line of an Ar+ ion laser at 45° and the photoluminescence (PL) was detected normal to the film surface. Typical PL spectra are depicted in Fig. 2a at a pH of and 3.1 and 11.2. As expected, the PL intensity of the thin films is dependent on the pH and is stronger under basic conditions. A typical titration curve is depicted in Fig. 2b. Compared to solution, the titration curve is relatively broad. This may be attributed to an inhomogeneous dye environment. Moreover, fluorescein possesses three pK values which can hardly be distinguished in solution (pK1 = 2.08, pK2 = 4.31, pK3 = 6.43) and which might also cause some broadening.10
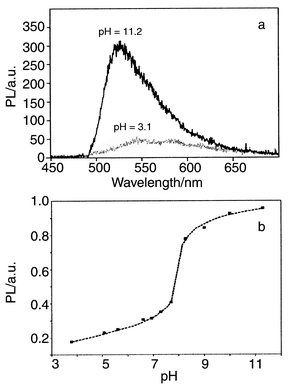 | ||
| Fig. 2 (a) Photoluminescence of fluorescein doped mesoporous thin films at low and high pH values, (b) typical titration curve. | ||
The response time of the mesoporous films upon changing the pH is fast, being in the range of a few seconds. Fig. 3 depicts a typical set of data which was obtained by exchanging the solution from pH 3.8 to 9.4. We attribute the fast response to the fact that the porous structure facilitates the diffusion of H3O+ (OH−). While it is possible the dye is located both at the pore interface and incorporated within the SiO2 wall, this does not hinder the anchored fluorescein entities to respond rapidly to changes in pH. From time dependent measurements we evaluated the time for the change in the PL signal to 95% of the final value to be in the range of ∼7 s. We note that with the exception of small capillaries coated with sol–gel glass,11 this is faster than in purely sol–gel based optical pH sensors.7,12
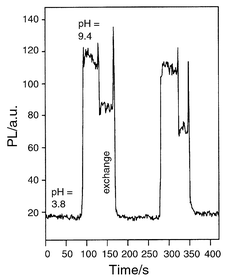 | ||
| Fig. 3 Time dependent photoluminescence response of a fluorescein doped mesoporous thin film upon change of the pH value. | ||
Here, we have demonstrated that dye-doped mesoporous thin films are suitable candidates for optical solid-state pH sensors. An important point is the careful optimization of the processing conditions which are necessary to obtain thin mesoporous films with high optical quality. As expected, the response time is very fast, due to the mesoporosity of the thin films. On the other hand, the high processibility of the solid state precursor solution should allow one to extend this chemistry to other sensing arrangements, e.g. to evanescent wave optical fibers13 or to pH sensing microtips.14
During review of this manuscript, Fau et al.15 reported pH sensing in patterned mesoporous materials where the sensing agent was attached by post-synthesis modification.
Acknowledgements
This work was supported by the NSF under grant DMR 95-20971, DMR 9634396, the U.S. Army Research Office under grant DAAH04-96-1-0443 and made use of the Materials Research central facilities supported by the NSF under award DMR-9632716.Notes and references
- P. Yang, G. Wirnsberger, H. C. Huang, S. R. Cordero, B. Scott, M. D. McGehee, T. Deng, G. M. Whitesides, B. F. Chmelka, S. K. Buratto and G. D. Stucky, Science, 2000, 287, 465 CrossRef CAS.
- F. Marlow, M. D. McGehee, D. Zhao, B. F. Chmelka and G. D. Stucky, Adv. Mater., 1999, 11, 632 CrossRef CAS.
- G. Wirnsberger and G. D. Stucky, Chem. Mater., 2000, 12, 2525 CrossRef CAS.
- B. Lebeau, C. F. Fowler, S. R. Hall and S. Mann, J. Mater. Chem., 1999, 9, 2279 RSC.
- e.g. A. K. McEvoy, C. M. McDonagh and B. D. MacCraith, Analyst, 1996, 121, 785 Search PubMed.
- e.g. B. Iosefzon-Kuyavskaya, I. Gigozin, M. Ottolenghi, D. Avnir and O. Lev, J. Non-Cryst. Solids, 1992, 147–148, 808 Search PubMed.
- C. Rottman, M. Ottolenghi, R. Zusman, O. Lev, M. Smith, G. Gong, M. L. Kagan and D. Avnir, Mater. Lett., 1992, 13, 293 CrossRef CAS.
- D. Zhao, P. Yang, N. Melosh, J. Feng, B. F. Chmelka and G. D. Stucky, Adv. Mater., 1998, 10, 1380 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chemlka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS; D. Zhao, Q. Huo, J. Feng, B. F. Chemlka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- R. Sjöback, J. Nygren and M. Kubista, Spectrochim. Acta A, 1995, 51, L7 CrossRef.
- J. Samuel, A. Strinkovski, S. Shalom, K. Lieberman, M. Ottolenghi, D. Avnir and A. Lewis, Mater. Lett., 1994, 21, 431 CrossRef CAS.
- L. Yang and S. S. Saavedra, Anal. Chem., 1995, 67, 1307 CrossRef CAS; A. Lobnik, I. Oehme, I. Murkovic and O. S. Wolfbeis, Anal. Chim. Acta, 1998, 367, 159 CrossRef CAS.
- O. Ben-David, E. Shafir, I. Gilath, Y. Prior and D. Avnir, Chem. Mater., 1997, 9, 2255 CrossRef CAS.
- W. Tan, R. Kopleman, S. L. R. Barker and M. T. Miller, Anal. Chem., 1999, 72, 606A.
- H. Fau, Y. Lu, A. Stump, S. T. Reed, T. Baer, R. Schunk, V. Perez-Lund, G. P. Lopez and C. J. Brinker, Nature, 2000, 405, 56 CrossRef.
| This journal is © The Royal Society of Chemistry 2001 |
