Tungsten–niobium–sulfur composite nanotubes†
Yan Qiu Zhua, Wen Kuang Hsua, Mauricio Terronesa, Steven Firthb, Nicole Groberta, Robin J. H. Clarkb, Harold W. Krotoa and David R. M. Waltona
aFullerene Science Centre, School of CPES, University of Sussex, Brighton, UK BN1 9QJ. E-mail: d.walton@sussex.ac.uk
bChristopher Ingold Laboratories, University College London, 20 Gordon St., London, UK WC1H 0AJ
First published on 18th December 2000
Abstract
Novel W–Nb–S composite inorganic nanotubes have been generated by replacing W in the S–W–S sandwich layers of WS2 by Nb. The tubes are uniform, with the Nb concentration 10 atom%. Raman spectral features of the tubes are described.
Inorganic fullerene (IF) nanotubes, e.g. MX2 (M = W, Mo; X = S, Se), first reported by Tenne et al.,1 have engendered considerable scientific interest because they are good semiconductors and display excellent wear-resistant properties. To date, various approaches to the synthesis of these materials have been developed, such as chemical transport,2 gas–solid reactions3 and in situ heating,4 leading to pure MX2 nanoparticles, short and long tubes, bundles, and even microtubes. The structural features, lubricating properties and electronic behaviour of the IF nanotubes have been widely investigated.5–8 For example, a single WS2 nanotube has been used successfully as a microscope probe.3,9 Recently, Levy and coworkers showed that small amounts of noble metals (Au and Ag) could be intercalated between WS2 tube walls.10,11 Akin to their bulk crystals, IF nanotube band gaps are dependent upon tube diameter.12 It is therefore interesting to prepare doped nanotubes and to assess their electronic and mechanical performance. Here, we describe the generation of a novel WxNbyS2 IF nanotube containing ca. 10 atom% Nb.
We prepared needle-like WOx (mainly W18O49) nanotubes by the previously described procedure.13 The needles were then covered with a suspension of Nb2O5 (Aldrich, 99.9% pure, particle size < 2 μm) in acetone, and the acetone was allowed to evaporate leaving Nb2O5 uniformly distributed over the needles. The sample was then reheated to ca. 1100 °C under 200 Torr H2S. Using a scalpel, the grey needle-like deposit was removed from the quartz surface, subjected to high resolution transmission electron microscopy (HRTEM, JEOL-4000) and to energy dispersive X-ray (EDX, element > B) and Raman spectroscopic (Renishaw system 1000, λ0 = 514.5 and 632.8 nm) analysis.
Previously, we had refined the oxide–sulfide conversion method7 and successfully created very long pure WS2 nanotubes by forming WOx nanorods prior to oxide–sulfide conversion.4 We have now found that the constituent atoms of Nb2O5-coated WOx nanorods easily diffuse at high temperature (ca. 1100 °C), particularly through materials possessing substantial vacancies. Thus Nb probably occupies some of the W positions within W18O49, leading to WxNbyOz. This replacement process is reminiscent of the behaviour of the bulk oxides, but in this case the nanorod templates and the suboxide features were retained. The presence of gaseous H2S in the chamber facilitated oxide-to-sulfide conversion in an identical manner to that found for the synthesis of pure WS2 or MoS2 nanotubes,1,3,4 resulting in homogeneous W–Nb–S composite nanotubes. These tubes are straight, ca. 5–10 μm long and ca. 20–100 nm in diameter. HRTEM images of the tubes are not different from those of WS2 nanotubes. The majority have hollow cores, less than 30% of them being fully or partly filled with elongated WOx crystals. Concentric nanotubes (layer separation ca. 0.62 nm) are observed. The outer layers are smooth and appear to be free of lattice defects. All the tubes are closed, ca. 20% by flat caps, forming a ca. 90-degree connection with the cylinders. Dislocations at the cap–cylinder junctions are apparent [Fig. 1(a), arrowed]. EDX revealed the presence of Nb [Fig. 1(b)] within the nanotubes. Measurements on ten isolated hollow nanotubes revealed a ca. 9∶1 W to Nb atomic ratio, the metal∶sulfur ratio being ca. 1∶2. The overall stoichiometry of the nanotubes is WxNbyS2 (x = 0.9, y = 0.1, 5% uncertainty). The flat tip feature (ca. 90-degree angle with the body) of the nanorods is determined by their intrinsic crystalline nature.13 Hence, the flat nanotube tips probably form as a result of oxide–sulfide template conversion. This conjecture is supported by supplementary experiments, which showed that NbS2 nanotubes could not be prepared directly from Nb2O5 powder alone. These results confirm that the suboxide plays a key role in the IF nanotube formation, and the process can be written in terms of eqns. (1) and (2):
| WOx (nanorod) + Nb2O5 (particle) → W0.9Nb0.1Ox (nanorod) (1) | (1) |
| W0.9Nb0.1Ox (nanorod) + H2S (gas)→ W0.9Nb0.1S2 (nanotube) + H2O (gas) (2) | (2) |
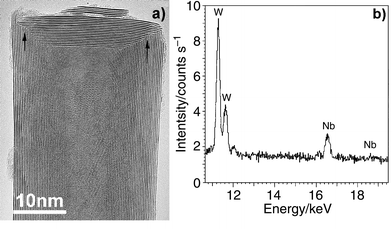 | ||
| Fig. 1 (a) HRTEM image showing a ca. 90-degree flat cap, layer separation = 0.62 nm. Dislocations at the cap-cylinder junctions are apparent (arrowed); (b) EDX of an isolated hollow tube, revealing the presence of W and Nb. S signals are omitted from this profile. | ||
Additional experiments have revealed that the W∶Nb ratio can be altered by applying various amounts of Nb2O5 coating to the WOx nanorods, prior to passage of H2S during heating. The Nb concentration (ca. 10% atom%) can be doubled in the nanotubes; however, our results showed that Nb-dominated W–Nb–S tubes are difficult to construct.
The Raman spectrum of W0.9Nb0.1S2 nanotubes taken with 632.8 nm excitation is identical to that of WS2 nanotubes. The 514.5 nm spectrum of the Nb-doped tubes, however, does show some differences from that of WS2 (Fig. 2), notably the appearance of a medium intensity band at 313 cm−1, a shoulder on the low wavenumber side of the e2g1 band at 356 cm−1 and an increase in the relative intensity of the 192 cm−1 band. The intensity of the 192 cm−1 band is ca. half that of the disorder-induced zone-edge phonon [LA(M)] band at 173 cm−1 for WS2, whereas its intensity for the Nb-doped tubes is ca. 5 times that of the 173 cm−1 band. The 192 cm−1 band has been observed in the Raman spectra of 2H-bulk and nanotubes of WS2, but has not been assigned.14 Its increase in relative intensity on going from pure WS2 nanotubes to Nb-doped WS2 nanotubes implies that it is caused by scattering from a disorder-induced zone-edge phonon. The band at 313 cm−1 has not previously been seen in the Raman spectra of any form of WS2, and is also probably due to the increased disorder in the lattice owing to the presence of Nb. The shoulder at 351 cm−1 is the first overtone of the LA(M) band, with its apparent intensity enhanced by its proximity to the strong band at 365 cm−1. The Raman bands and their assignments are summarised in Table 1 of ESI.†
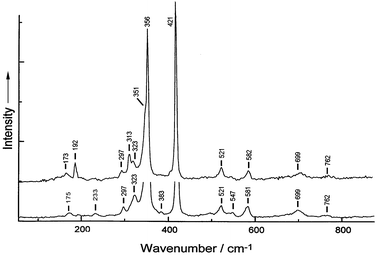 | ||
| Fig. 2 Raman spectra (λ0 = 514.5 nm) of W0.9Nb0.1S2 (upper) and WS2 (lower) nanotubes. | ||
The formation of W0.9Nb0.1S2 nanotubes can be accounted for by considering the layered structure of the W0.9Nb0.1S2 crystals. According to our EDX analysis, only ca. 10% of the W atoms are replaced by Nb. Therefore, we suggest that the Nb-doped material remains as the 2Hb-type WS2 structure, rather than becoming the 2Ha-type NbS2 structure. We propose a model for layered W0.9Nb0.1S2 (Fig. 3). In this structure, the doped Nb atoms (medium circles) should not cause severe lattice defects and can easily be stabilised by neighbouring atoms. This suggestion is supported by our Raman spectra; the spectra of the Nb-doped and pure WS2 tubes are identical using 632.8 nm excitation, whereas new bands attributable to increased lattice disorder are seen using 514.5 nm excitation. The crystal lattice spacing, particularly c/2, remains almost unchanged when compared with that of pure WS2 layers. If a nanotube is to be formed by rolling up such composite layers, one would not expect a significant c/2 change. This conjecture is also supported by the facts that the composition of our nanotubes is uniform and that we did not observe severe defects on the tube walls.
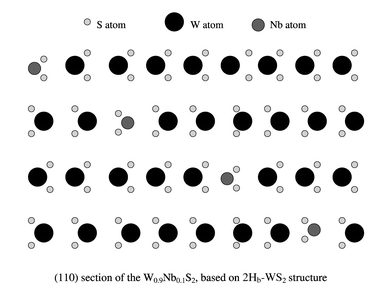 | ||
| Fig. 3 Proposed model for the layered W0.9Nb0.1S2 structure. | ||
Acknowledgements
We thank the Royal Society, the JFCC, the EPSRC and the ULIRS, for financial support. We are grateful to J. Thorpe and D. Randall (Sussex) for assistance with TEM and SEM facilities. We thank M. Mayne and I. Maurin for helpful discussions.Notes and references
- R. Tenne, L. Margulis, M. Genut and G. Hodes, Nature, 1992, 360, 444 CrossRef CAS.
- M. Remskar, Z. Skraba, M. Regula, C. Ballif, R. Sanjines and F. Levy, Adv. Mater., 1998, 10, 246 CrossRef CAS.
- M. Homyonfer, B. Alperson, Y. Rosenberg, L. Sapir, S. R. Cohen, G. Hodes and R. Tenne, J. Am. Chem. Soc., 1997, 119, 2693 CrossRef CAS.
- Y. Q. Zhu, W. K. Hsu, N. Grobert, B. H. Chang, M. Terrones, H. Terrones, H. W. Kroto, D. R. M. Walton and B. Q. Wei, Chem. Mater., 2000, 12, 1190 CrossRef CAS.
- J. Sloan, J. H. Hutchison, R. Tenne, Y. Felfman, T. Tsirlina and M. Homyonfer, J. Solid State Chem., 1999, 144, 100 CrossRef CAS.
- M. Remskar, Z. Skraba, R. Sanjines and F. Levy, Appl. Phys. Lett., 1999, 74, 3633 CrossRef CAS.
- L. Rapoport, Y. Bilik, Y. Feldman, M. Homyonfer, S. R. Cohen and R. Tenne, Nature, 1999, 387, 791.
- G. Seifert, H. Terrones, M. Terrones, G. Jungnickel and T. Frauenheim, Solid State Commun., 2000, 114, 245 CrossRef CAS.
- A. Rothschild, S. R. Cohen and R. Tenne, Appl. Phys. Lett., 1999, 75, 4025 CrossRef CAS.
- M. Remskar, Z. Skraba, C. Ballif, R. Sanjines and F. Levy, Surf. Rev. Lett., 1999, 6, 1283 CrossRef CAS.
- M. Remskar, Z. Skraba, P. Stadelmann and F. Levy, Adv. Mater., 2000, 12, 814 CrossRef CAS.
- G. Seifert, H. Terrones, M. Terrones, G. Jungnickel and T. Frauenheim, Phys. Rev. Lett., 2000, 85, 146 CrossRef CAS.
- Y. Q. Zhu, W. B. Hu, W. K. Hsu, M. Terrones, N. Grobert, H. Terrones, H. W. Kroto and D. R. M. Walton, Chem. Phys. Lett., 1999, 309, 327 CrossRef CAS.
- G. L. Frey, R. Tenne, M. J. Matthews, M.S. Dresselhaus and G. Dresselhaus, J. Mater. Res., 1998, 13, 2412 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: HRTEM image of a typical 10-layered W0.9Nb0.1S2 nanotube, layer separation = 0.62 nm and Raman bands of W0.9Nb0.1S2 and WS2 nanotubes and their assignments. See http://www.rsc.org/suppdata/cc/b0/b007074m/ |
| This journal is © The Royal Society of Chemistry 2001 |
