Pd-catalysed arylation of propan-1-ol and derivatives: oxidative role of the arylating agent
Laurence
Bagnell
,
Ulf
Kreher
and
Christopher R.
Strauss
*
CSIRO Molecular Science, Private Bag 10, Clayton South, Victoria 3169, Australia.. E-mail: chris.strauss@molsci.csiro.au
First published on 18th December 2000
Abstract
With excess PhI under Pd catalysis, 1-PrOH was converted to a mixture of 3,3-diphenylpropenal and trans-2,3-diphenylpropenal by a concerted, oxidative sequence that involved two arylative couplings and an olefinic aldehyde that was generated in situ.
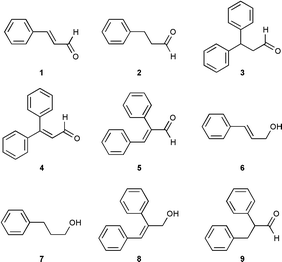
Recently in our laboratory, Pd on porous glass was developed as a heterogeneous catalyst1 for promoting Glaser-type and Heck arylative couplings2 without the need for solubilising or activating ligands. Significantly, with excess allyl alcohol and with PhI in air, Pd on porous glass gave trans-cinnamaldehyde (1) along with 3-phenylpropanal (2; see entry 2, Table 1).1 Consistent with literature reports,3,4 in the absence of air, and without the addition of Ag+ salts, 2 predominated and 1 was not obtained (entry 1, Table 1).
| Entry | Starting material (mmol) | PhI/mmol | Ar or Air | Major products (percentage of total detected) | Turnover Number |
|---|---|---|---|---|---|
| 1 | CH2![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH–CH2OH
(13) CH–CH2OH
(13) |
6 | Ar | 2 (59), CH3CH(Ph)CHO (23) | 170 |
| 2 | CH2![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH–CH2OH
(13) CH–CH2OH
(13) |
6 | Air | 1 (60), 2 (40) | 120 |
| 3 | CH2![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH–CH2OH
(14) CH–CH2OH
(14) |
34 | Air | 1 (16), 2 (7), 3 (24), 4 (26) | 450 |
| 4 | PhCH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH–CHO, 1 (13) CH–CHO, 1 (13) |
34 | Ar or Air | 4 (99) | 245 (Ar), 170 (Air) |
| 5 | PhCH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHCH2OH, 6 (13) CHCH2OH, 6 (13) |
34 | Ar | 3 (36), 4 (18), 8 (15), 9 (10) | 700 |
| 6 | PhCH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHCH2OH, 6 (13) CHCH2OH, 6 (13) |
34 | Air | 3 (6), 4 (62), 8 (13) | 560 |
| 7 | PhCH2CH2CHO, 2 (6) | 34 | Ar | 1 (5), 3 (16), 4 (39), 5 (6), 9 (18) | 270 |
| 8 | PhCH2CH2CH2OH, 7 (10) | 34 | Ar | 4 (60), 5 (18) | 50 |
| 9 | CH3CH2CH2OH (20) | 34 | Ar | 4 (80), 5 (18) | 30 |
With PhI in excess, the diarylated compounds 3 and 4 appeared as major products from allyl alcohol, along with 1 and 2 (entry 3, Table 1). This diarylation appeared to contrast with a wealth of literature data on the Pd catalysed arylation of that alcohol.3 Without Ag+ salts,4 one might expect that the first arylation of allyl alcohol would be accompanied by rapid migration of the olefinic bond to afford mainly 3-phenylpropanal (2), thereby removing the opportunity for a second Heck arylation to occur.
Although air facilitated the dehydrogenation for the reaction in entry 2 of Table 1,5 the results in entry 3 and co-formation of biphenyl suggested that the arylating agent may have had an additional role. To support this contention, we now report that 1-PrOH with excess PhI can afford a mixture of 3,3-diphenylpropenal (4) and trans-2,3-diphenylpropenal (5), even under an inert gas atmosphere (see entry 9). This remarkable transformation has been performed with either Pd(OAc)2 or Pd on porous glass as catalyst6,7 (which was used for all entries in Table 1) includes microwave heating8 in a reactor of our design.9
A comparable stepwise process would require oxidation of the hydroxy function of 1-PrOH, dehydrogenation of the hydrocarbon chain and two Heck-like arylative couplings of the resultant olefin. However, for the one reaction, successive intermolecular Heck arylative couplings onto the same olefin are rare.10 Intramolecular examples usually have involved mono-arylations of more than one carbon–carbon double bond.11
Mono-arylated products were not observed in the reaction of 1-PrOH, rendering as unlikely, a multi-step sequence involving successive intermolecular Heck arylative couplings with an olefin formed by dehydrogenation in situ.12 The absence of detectable intermediates implies that the process was concerted and may constitute a new reaction.
Formation of biphenyl and benzene as by-products indicates that PhI served as both an oxidant and a reactant. Buchwald and Palucki observed similar behaviour of their arylating agent in the ‘Pd’ catalysed reaction of cyclohexanol with 4-tert-butylbromobenzene to produce the corresponding α-arylated cyclohexanone and tert-butylbenzene.13 They surmised that some of the haloarene was simultaneously reduced during oxidation of the alcohol to cyclohexanone.
Although variations in the order of transformations and alternative routes are possible for the present reaction, the pathway in Scheme 1 appears to account for the products, by-products and their relative proportions.
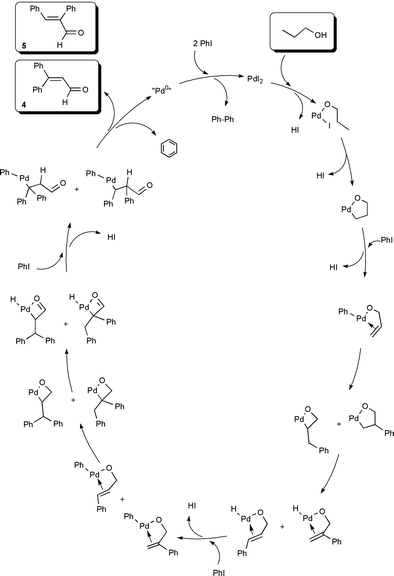 | ||
| Scheme 1 | ||
Preliminary investigations (see Table 1) into the process utilised cinnamaldehyde (1),14 cinnamyl alcohol (6), 3-phenylpropanol (7) and 3-phenylpropanal (2). Cinnamyl alcohol (6) afforded diarylenal 4 as a major product (along with 3 and the 2,3-diphenylpropenol isomer 8), irrespective of whether or not the atmosphere contained air (entries 5 and 6). Under argon, 3-phenylpropanal (2) gave 2,3-diphenylpropanal (9) and products of higher oxidation state, including 1, 4 and 5 (entry 7). 3-Phenylpropanol (7) afforded 4 and 5 as major products, along with traces of 1 and 2 (entry 8). The product distributions obtained from Pd catalysed reactions of excess PhI with 6, 7 and 2 suggest that several competing processes were operating, including α-arylation,13 Heck-like arylative coupling of olefinic bonds2 as well as the new process described herein and illustrated by Scheme 1.
These outcomes may have depended on whether or not coordination of the olefin to Pd occurred in conjunction with the establishment of a strong Pd–O bond, leading to a chelation controlled reaction. Such bonds have been suggested for palladium catalysed oxidation of alcohols.15Scheme 1, proposed for the reaction of 1-PrOH commences with Pd–O chelation and proceeds preferably through metallaoxetane intermediates,16 which account for the observed products and their relative distribution. In the case of allyl alcohol (entry 3), it appears that the double bond and the hydroxy group of the alcohol compete for the Pd and products from both Heck-type arylative coupling (2 and 3) as well as the sequence in Scheme 1 (diarylenal 4) result.
Acknowledgements
We thank Professor A. Hallberg and Dr M. Larhed of Uppsala University, Sweden, and Dr H. Weigold of CSIRO for reviewing drafts of the manuscript and for helpful discussion. The work was supported by a postdoctoral fellowship (for U.K.) from the German Academic Exchange Service (DAAD).Notes and references
- J. Li, A. W.-H. Mau and C. R. Strauss, Chem. Commun., 1997, 1275 RSC.
- A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379 CrossRef; R. F. Heck in Comprehensive Organic Synthesis, series eds. B. M. Trost, I. Fleming, vol. ed. M. F. Semmelhack, Pergamon, Oxford, 1993, vol. 4, p. 833. Search PubMed.
- J. B. Melpolder and R. F. Heck, J. Org. Chem., 1976, 41, 265 CrossRef CAS; A. J. Chalk and S. A. Magennis, J. Org. Chem., 1976, 41, 273 CrossRef CAS; A. J. Chalk and S. A. Magennis, J. Org. Chem., 1976, 41, 1206 CrossRef; T. Jeffrey, J. Chem. Soc., Chem. Commun., 1984, 1287 RSC; R. C. Larock, W.-Y. Leung and S. Stolz-Dunn, Tetrahedron Lett., 1989, 30, 6629 CrossRef CAS; S.-K. Kang, K.-Y. Jung, C.-H. Park, E.-Y. Namkoong and T.-H. Kim, Tetrahedron Lett., 1995, 36, 6287 CrossRef CAS; G. Dyker and P. Grundt, Tetrahedron Lett., 1996, 37, 619 CrossRef CAS.
- T. Jeffery, Tetrahedron Lett., 1991, 32, 2121 CrossRef CAS.
- C. R. Strauss, Aust. J. Chem., 1999, 52, 83 CAS.
- A Into a dry, two-necked flask fitted with a reflux condenser and an inert gas (Ar) bleed line was placed a solution of 1-PrOH (0.784 g; 13.06 mmol) and PhI (12.674 g; 62.12 mmol) in dry, deoxygenated N,N-dimethyl acetamide (DMA; 50 mL). Anhydrous NaOAc (5.80 g; 70.70 mmol) and Pd(OAc)2 (0.564 g; 2.51 mmol) were added and the mixture was heated at 125 °C for 16 h, then cooled, quenched with 80 mL H2O and extracted with Et2O (1 × 80 mL, 2 × 50 mL). The ether extract was washed thrice with water, dried with MgSO4 and the ether removed in vacuo. Unconverted PhI was recovered by vacuum distillation at 40 °C and 1 × 10−2 mbar and biphenyl was removed by sublimation (80 °C and 1 × 10−2 mbar). Flash chromatography (silica gel 60, dichloromethane∶pentane 1:1) afforded pure product 4 (395 mg). B Into a dry, two-necked flask fitted with a reflux condenser and an inert gas (Ar) bleed line was placed a solution of 1-PrOH (749 mg; 12.48 mmol) and PhI (6.971 g; 34 mmol) in dry, deoxygenated N,N-dimethyl acetamide (DMA; 50 mL). Anhydrous NaOAc (4.127 g; 50 mmol) and Pd on porous glass (containing 19 μmol Pd)1 were added and the mixture was heated at 125 °C for 16 h, then cooled. Work up as above yielded pure product 4 (73 mg)..
- Although Pd(OAc)2 undergoes thermal decomposition to give finely divided Pd metal and gases including CO2, methane and ethane (see M. T. Reetz and M. Maase, Adv. Mat., 1999, 11, 773; M. T. Reetz and G. Lohmer, Chem. Commun., 1996, 1921), no evidence was obtained to associate that redox process with the present reaction..
- Into a dry PTFE vessel under nitrogen was placed a solution of 1-PrOH (0.803 g; 13.4 mmol) and PhI (6.853 g; 33.6 mmol) in dry, deoxygenated N,N-dimethyl acetamide (DMA; 50 mL). Anhydrous NaOAc (4.19 g; 51.0 mmol) and Pd(OAc)2 (50.4 mg; 0.22 mmol) were added and the mixture was heated for 10 min at 220 °C in a microwave batch reactor9 (MBR), then cooled. Work up as in footnote 6 yielded pure product 4 (280 mg). For other examples of Pd catalysed reactions under microwave heating, see M. Larhed and A. Hallberg, J. Org. Chem., 1996, 61, 9582 CrossRef CAS; U. Bremberg, M. Larhed, C. Moberg and A. Hallberg, J. Org. Chem., 1999, 64, 1082 CrossRef CAS; M. Larhed, M. Hoshino, S. Hadida, D. P. Curran and A. Hallberg, J. Org. Chem., 1997, 62, 5583 CrossRef CAS.
- C. R. Strauss and R. W. Trainor, Aust. J. Chem., 1995, 48, 1665 CrossRef CAS; K. D. Raner, C. R. Strauss, R. W. Trainor and J. S. Thorn, J. Org. Chem., 1995, 60, 2456 CrossRef CAS.
- For examples see T. Sugihara, M. Takebayashi and C. Kaneko, Tetrahedron Lett., 1995, 36, 5547 CrossRef CAS; B. M. Choudary, R. M. Sarma and K. K. Rao, Tetrahedron, 1992, 48, 719 CrossRef CAS.
- L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS; M. Lautens and S. Piguel, Angew. Chem., Int. Ed., 2000, 39, 1045 CrossRef CAS.
- In water at high temperature, Pd(OAc)2 catalysed Heck arylations have been reported with olefins generated in situ. However, at 400 °C, the reaction between PhI and 1-PrOH gave 1-propylbenzene by a mechanism that was not determined. See E. J. Parsons, CHEMTECH, 1996, 26(7), 30..
- M. Palucki and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 11108 CrossRef CAS.
- A. Amorese, A. Arcadi, E. Bernocchi, S. Cacchi, S. Cerrini and W. Fedeli and G. Ortar, Tetrahedron, 1989, 45, 813 CrossRef CAS.
- S. Ait-Mohand, F. Henin and J. Muzart, Tetrahedron Lett., 1995, 36, 2473 CrossRef; V. Bellosta, R. Benhaddou and S. Czernecki, Synlett, 1993, 861 CrossRef CAS.
- E. Bernocchi, S. Cacchi, P. G. Ciattini, E. Morera and G. Ortar, Tetrahedron Lett., 1992, 33, 3073 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |