The effect of solvent on the α-effect:
C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/h2_char_e006.gif) O, P
O, P![[double bond, length half m-dash]](https://www.rsc.org/images/entities/h2_char_e006.gif) O and SO2 centers†
O and SO2 centers†
Ik-Hwan
Um
*a,
Jin-Young
Hong
a and
Erwin
Buncel
*b
aDepartment of Chemistry, Ewha Womans University, Seoul, 120-750, Korea
bDepartment of Chemistry, Queen’s University, Kingston, Ontario K7L 3N6, Canada.. E-mail: ihum@mm.ewha.ac.kr;; Fax: (+1)-(822)-3277-2844;; Tel: (+1)-(822)-3277-2349
First published on 11th December 2000
Abstract
The α-effect for the reaction of a sulfonyl ester exhibits a bell-shaped dependence of the α-effect on solvent composition as do the corresponding reactions with a carbonyl and a phosphinyl ester, and the magnitude of the α-effect is found to be dependent on the magnitude of the βnuc value, suggesting TS stabilization as the cause of the α-effect.
Since the α-effect term was given by Edwards and Pearson in 1962 to the abnormally enhanced reactivity shown by nucleophiles having a pair of unshared electrons adjacent to the nucleophilic center,1 numerous studies have been performed to account for this phenomenon.2–11 However, the cause of the α-effect has not been clearly understood.2–11 One of the intriguing aspects in α-effect studies has been the finding that the magnitude of the α-effect is dependent on the nature of the electrophilic center, generally increasing as sp3 < sp2 < sp for carbon centers,2–4 though some exceptions exist.5 Equally interesting, as well as controversial, has been the effect of solvent on the α-effect.6–9
In order to shed light on the effect of solvent on the α-effect, we initiated systematic studies and in 1986, we investigated the reaction of p-nitrophenyl acetate (PNPA) with butane-2,3-dione monoximate (Ox−) and p-chlorophenoxide (ClPhO−), as the α- and corresponding normal-nucleophile, respectively, in DMSO–H2O mixtures of varying compositions.6a We found, unexpectedly, a bell-shaped dependence of the α-effect (kOx−/kClPhO −) on solvent composition.6a A similar bell-shaped trend was observed for the corresponding reaction of p-nitrophenyl diphenylphosphinate (PNPDPP).6b However, Moss reported that the reaction of PNPA with o-iodosylbenzoate (IBO−) and ClPhO− in DMSO–H2O mixtures shows no maximum α-effect but exhibits a decreasing α-effect trend.7 More surprisingly, a contrasting solvent behaviour was found recently:8 the reaction of PNPA with Ox− and ClPhO− in MeCN–H2O mixtures exhibits an increasing α-effect trend as the mol% MeCN in the medium is increased.8a Similarly, the α-effect for the reaction of PNPA with benzohydroxamates and m-chlorophenoxide in MeCN–H2O mixtures also resulted in an increasing α-effect behaviour as the concentration of MeCN in the reaction medium was increased.8b
It appeared to us as potentially highly informative, in investigation of the α-effect, to vary the electrophilic center systematically and to couple that with variation of solvent. We report herein such a study for the reaction of a sulfur centered substrate, p-nitrophenyl benzenesulfonate (PNPBS), with Ox− and ClPhO− in DMSO–H2O mixtures as shown in eqn. (1), and compare the results with the data for the corresponding reactions of PNPA and PNPDPP.
C6H5SO2-OC6
H4NO2-p + Nu−
→C6H5SO2Nu +
−OC6H4NO2-p
Nu− =
CH3C(O)C(CH3)![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) NO−
(Ox−), an
α-nucleophilep-ClC6H4O−
(ClPhO−), a normal nucleophile NO−
(Ox−), an
α-nucleophilep-ClC6H4O−
(ClPhO−), a normal nucleophile | (1) |
As shown in Fig. 1, the second-order rate constant increases as the mol% of DMSO in the medium increases for both Ox− and ClPhO− systems: the rate enhancement upon solvent change from 10 to 90 mol% of DMSO is 2190 and 3330 for Ox− and ClPhO−, respectively. Interestingly, the plot of log kOx−vs. mol% of DMSO shows downward curvature, while that of log kClPhO−vs. mol% DMSO exhibits upward curvature. As a result, the difference in rate constant between Ox− and ClPhO− increases up to ca. 50 mol% DMSO but decreases beyond this point. Such a differential solvent effect on rates leads to the solvent dependent α-effect profile, shown in Fig. 2; i.e. the sulfonyl system exhibits maximum α-effect at ca. 50 mol% DMSO, as do the carbonyl and phosphinyl systems. Therefore, the bell-shaped α-effect behaviour has been found to be general for the reactions of the three different electrophiles with Ox− and ClPhO− in DMSO–H2O mixtures.
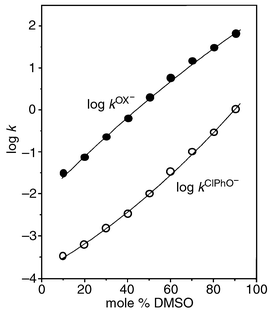 | ||
| Fig. 1 Plots showing the effect of solvent on second-order rate constants for the reaction of PNPBS with Ox− and ClPhO− in DMSO–H2O mixtures at 25.0 ± 0.1 °C. | ||
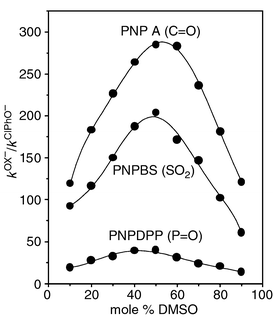 | ||
| Fig. 2 Plots showing the effect of solvent on the α-effect for the reaction of PNPA, PNPDPP and PNPBS with Ox− and ClPhO− in DMSO–H2O mixtures at 25.0 ± 0.1 °C. | ||
Moreover, interestingly, the magnitude of the α-effect is strongly dependent on the electrophilic center; i.e. the α-effect in 50 mol% is ca. 300, 40 and 200 for the carbonyl, phosphinyl and sulfonyl systems, respectively. The small α-effect for the phosphinyl system compared to the carbonyl and sulfonyl systems is striking. Bruice showed that the magnitude of the α-effect is dependent on the magnitude of the βnuc value for reactions of a variety of substrates with hydrazine and glycylglycine: the α-effect decreases with decreasing βnuc value.10 Similarly, Bernasconi observed no α-effect for the addition reaction of primary amines including hydrazine and o-methylhydroxylamine to Meldrum’s acid, a system for which βnuc = 0.22.11a
The βnuc values for the reactions of PNPA with substituted phenoxides in various DMSO–H2O mixtures are available,12 but the ones for the reaction of the phosphinyl and sulfonyl systems have not been reported. Therefore, we performed the reaction of PNPDPP and PNPBS with a series of substituted phenoxides in 50 mol% DMSO, in which the maximum α-effect is observed. The plots of log kZC6H4O−vs.pKa (ZC6H4OH) exhibit good Brønsted type correlation: βnuc values are 0.64, 0.21 and 0.54 for the carbonyl, phosphinyl and sulfonyl systems, respectively. Thus, the βnuc value for the phosphinyl systems is much smaller than for the carbonyl and sulfonyl systems, and, moreover, the βnuc value follows the same order as the α-effect in magnitude. Therefore, one can suggest that the small βnuc value is responsible for the small α-effect exhibited by the phosphinyl system. This argument is consistent with our recent report that the reaction for an sp hybridized carbon center exhibited an unexpectedly small α-effect in which the βnuc value was 0.32.5
As demonstrated in Fig. 2, the effect of solvent on reactivity is significant. Such a solvent effect on rate can be achieved by destabilizing the ground-state (GS) and/or stabilizing the transition-state (TS). We recently found that the GS of Ox− and ClPhO− becomes destabilized upon addition of DMSO to the reaction medium,6c however, the GS energy difference between Ox− and ClPhO− is constant for the three systems. Therefore, if the GS energy difference between Ox− and ClPhO− were mainly responsible for the α-effect, the magnitude of the α-effect should be about the same, regardless of the nature of the electrophilic center. However, our results show that this is not the case. Therefore, the present results clearly suggest that the difference in the GS energy is not solely responsible for the α-effect.
The magnitude of the βnuc value has been understood as a measure of bond formation between the nucleophile and the substrate in the TS of the rate-determining step; hence the TS structures of the carbonyl, phosphinyl and sulfonyl systems would vary according to the different βnuc values. One can expect that the TS stabilizing effect would be smaller for the reaction system in which the degree of bond formation between nucleophile and substrate in the TS is less advanced (reactant-like TS), and vice versa. Accordingly, one can suggest that the TS stabilizing effect would be developed to a lesser extent for the phosphinyl system compared with the carbonyl and sulfonyl systems, based on the smaller βnuc value obtained for the former, which would explain the small α-effect observed for the phosphinyl system.
More systematic studies are underway including theoretical investigation for better understanding of solvent effect on the α-effect.
Acknowledgements
The authors are grateful for the financial support from KOSEF of Korea (1999-2-123-003-5 and 2000-123-02-2) and E. B. also thanks NSERC of Canada for a research grant.Notes and references
- J. O. Edwards and R. G. Pearson, J. Am. Chem. Soc., 1962, 84, 16 CrossRef CAS.
- Reviews: (a) E. Buncel and S. Hoz, Isr. J. Chem., 1985, 26, 313 Search PubMed; (b) A. P. Grekov and V. Y. Veselov, Usp. Khim., 1978, 47, 1200 Search PubMed; (c) N. J. Fina and J. O. Edwards, Int. J. Chem. Kinet., 1973, 5, 1 CrossRef.
- (a) G. Moutiers, E. Guevel, L. Villien and F. Terrier, J. Chem. Soc., Perkin Trans. 2, 1997, 710 Search PubMed; (b) F. Terrier, G. Moutiers, L. Xiao, E. Guevel and F. Guir, J. Org. Chem., 1995, 60, 1748 CrossRef CAS; (c) K. R. Fountain, D. B. Tad-y, T. W. Paul and M. V. Golynskiy, J. Org. Chem., 1999, 64, 6547 CrossRef CAS.
- (a) E. Buncel, C. Chuaqui and H. Wilson, J. Am. Chem. Soc., 1982, 104, 4896 CrossRef CAS; (b) S. Oae and Y. Kadoma, Phosphorus, Sulfur Silicon Relat. Elem., 1997, 123, 293 Search PubMed.
- I. H. Um, J. S. Lee and S. M. Yuk, J. Org. Chem., 1998, 63, 9152 CrossRef CAS.
- (a) E. Buncel and I. H. Um, J. Chem. Soc., Chem. Commun., 1986, 595 RSC; (b) R. M. Tarkka and E. Buncel, J. Am. Chem. Soc., 1995, 117, 1503 CrossRef CAS; (c) I. H. Um and E. Buncel, J. Org. Chem., 2000, 65, 577 CrossRef CAS.
- R. A. Moss, S. Swarup and S. Ganguli, J. Chem. Soc., Chem. Commun., 1987, 860 RSC.
- (a) I. H. Um and E. Buncel, J. Chem. Soc., Chem. Commun., 2000, 1917 RSC; (b) I. H. Um, H. W. Yoon, J. S. Lee and H. J. Moon, J. Org. Chem., 1997, 62, 5939 CrossRef CAS.
- (a) G. Moutiers, E. Guevel, L. Villien and F. Terrier, J. Chem. Soc., Perkin Trans. 2, 1997, 7 RSC; (b) C. A. Bunton, N. D. Gillitt and A. Kumar, J. Phys. Org. Chem., 1997, 10, 221 CrossRef CAS; (c) M. Laloi-Diard, J. F. Verchere, P. Gosselin and F. Terrier, Tetrahedron Lett., 1984, 25, 1267 CrossRef CAS.
- H. J. Dixon and T. C. Bruice, J. Am. Chem. Soc., 1972, 94, 2052 CrossRef.
- (a) C. F. Bernasconi and C. Murray, J. Am. Chem. Soc., 1986, 108, 5251 CrossRef CAS; (b) D. Herschlag and W. P. Jencks, J. Am. Chem. Soc., 1990, 112, 1951 CrossRef CAS; (c) L. E. Fikes, D. T. Winn, R. W. Sweger, M. P. Johnson and A. W. Czarnik, J. Am. Chem. Soc., 1992, 114, 1493 CrossRef CAS.
- E. Buncel, I. H. Um and S. Hoz, J. Am. Chem. Soc., 1989, 111, 971 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables of rate constants. See http://www.rsc.org/suppdata/cc/b0/b007000i/ |
| This journal is © The Royal Society of Chemistry 2001 |
