Grignard reagent mediated reaction of Cp2Zr(II)–ethylene complex with imines†
Tamotsu
Takahashi
*,
Yuanhong
Liu
,
Chanjuan
Xi
and
Shouquan
Huo
Catalysis Research Center and Graduate School of Pharmaceutical
Sciences, Hokkaido University; and CREST, Science and Technology Corporation
(JST), Sapporo, 060-0811, Japan.. E-mail: tamotsu@cat.hokudai.ac.jp
First published on 11th December 2000
Abstract
Imines which do not react with Grignard reagents reacted with EtMgBr in the presence of a catalytic amount of Cp2ZrCl2 to give ethylated products in excellent yields; the stoichiometric reaction of the imines and the zirconocene–ethylene complex did not give the ethylated product, whereas addition of MeMgBr or BuMgCl to the mixture afforded the ethylated product after hydrolysis.
We have found that a zircononcene–ethylene complex Cp2Zr(CH2
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2) (2)1 and its ate complex
[Cp2ZrEt(CH2
CH2) (2)1 and its ate complex
[Cp2ZrEt(CH2![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2)]MgBr2 prepared from Cp2ZrEt2
(1) and zirconacyclopentanes1 were
involved in the zirconium-catalysed reaction of olefins with EtMgBr3 which was first reported by Dzhemilev.4 In order to extend this type of reaction we
investigated a catalytic and a stoichiometric reaction of imine with EtMgBr
in the presence of zirconocene. In this paper we would like to report a
zirconium-catalysed reaction of imine with EtMgBr and the unusual effect of
addition of Grignard reagents on the formation of a new C–C bond in
the stoichiometric reaction of imines with the zirconocene–ethylene
complex.
CH2)]MgBr2 prepared from Cp2ZrEt2
(1) and zirconacyclopentanes1 were
involved in the zirconium-catalysed reaction of olefins with EtMgBr3 which was first reported by Dzhemilev.4 In order to extend this type of reaction we
investigated a catalytic and a stoichiometric reaction of imine with EtMgBr
in the presence of zirconocene. In this paper we would like to report a
zirconium-catalysed reaction of imine with EtMgBr and the unusual effect of
addition of Grignard reagents on the formation of a new C–C bond in
the stoichiometric reaction of imines with the zirconocene–ethylene
complex.
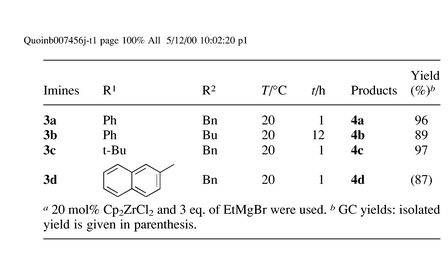
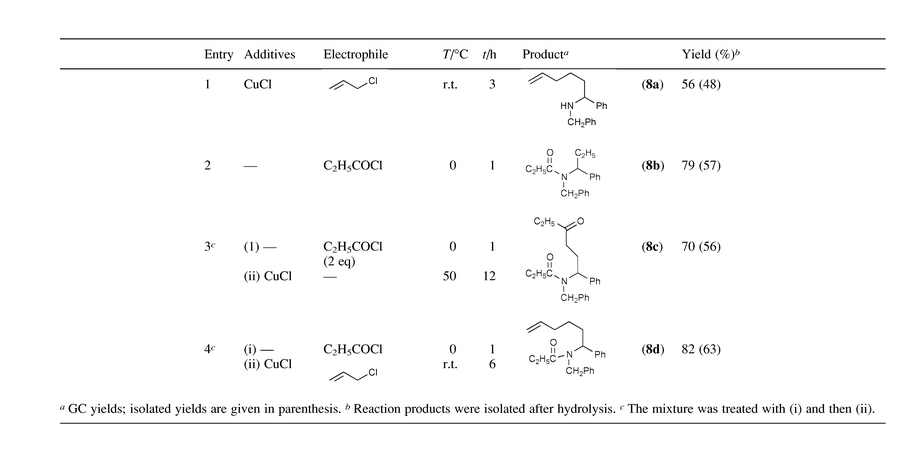
Imines 3a–d, easily prepared from the corresponding aldehydes and amines, do not react with EtMgBr in THF at even 50 °C to give addition products. When 20 mol% Cp2ZrCl2 was added to the mixture of the imines and EtMgBr in THF, the reaction proceeded smoothly at rt to produce substituted amine derivatives 4 in excellent yields after hydrolysis. The results are shown in Table 1. At least 3 eq. of EtMgBr was needed to finish the reaction, and the use of 10 mol% of Cp2ZrCl2 decreased the yield of products 4 [eqn. (1)].
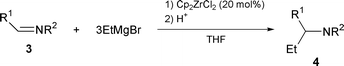 | (1) |
We investigated the stoichiometric reactions of imines with the zirconocene–ethylene complex. And quite surprisingly, we found that the stoichiometric reaction of 3c with 2 did not give the coupling product (only 7% of 4c) after hydrolysis. It puzzled us, however, that when an additional amount of EtMgBr (1 eq.) was added to the reaction mixture, the desired product 4c was obtained in 64% yield. Moreover, addition of 4 eq. of EtMgBr to the mixture gave an excellent yield of 4c (93%) [eqn. (2)].
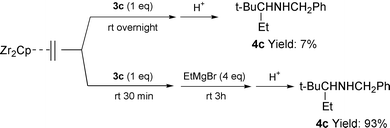 | (2) |
It is noteworthy that the use of different Grignard reagents also afforded only the same ethylated product 4c, with improved yields as shown in Table 2.
It is interesting that the reaction of 3a with 2 gave the ethylated product 4a in 57% yield after hydrolysis. This indicates that the azazirconacylopentane 5a was formed in the case of 3a. A similar effect of addition of a Grignard reagent was also observed. In fact, addition of 1 eq. of MeMgBr to the mixture of 3a and 2 afforded 4a in 75% yield after hydrolysis. When 4 eq. of EtMgBr was added to the mixture of 3a and 2, the product 4a was formed in 97% yield after hydrolysis.
These results suggest that there is an equilibrium between
azazirconacyclopentane 5 and imine 3 and that the
position of the equilibrium lies to the left under the conditions used
here. If an irreversible step such as ring-opening and β-hydrogen
abstraction is involved in the reaction, in other words, when additional
Grignard reagents are added, the reaction goes to the right as expected to
give the ethylated products. Deuterolysis instead of hydrolysis of the
reaction mixture after addition of 4 eq. of MeMgBr to a mixture of
3c and 2 afforded deuterated product 4c
DCH2CH2(t-Bu)CHNDCH2Ph (65% D) [eqn. (3)].
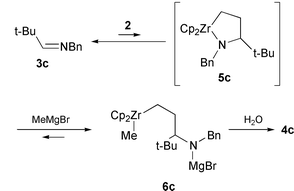
This strongly suggests that the complex 6c is the reaction product
of MeMgBr with a mixture of 3c and 2. When 6c
was treated with 2 eq. of CuCl for 6 h at rt, ethylated imine
tBu(Et)CHN![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHPh (7) was obtained in 48% yield after
hydrolysis.
CHPh (7) was obtained in 48% yield after
hydrolysis.
We also carried out the reactions of a mixture of Cp2ZrEt2 and imine 3a with various electrophiles5,6 and the results are summarized in Table 3.
 | (3) |
We have developed several zirconium-catalyzed reactions, in which a zirconocene–olefin complex acts as the key catalytic species. In the light of our previous work, a plausible mechanism for this catalytic addition of EtMgBr to imines is shown in Scheme 1, which involves: (1) generation of zirconocene–ethylene complex 2 from Cp2ZrCl2 and two eq. of EtMgBr; (2) coupling with imine 3 to form azazirconacyclopentane 5; (3) ring-opening reaction by EtMgBr; and (4) β-elimination to regenerate zirconocene–ethylene complex 2 and to release metalated amine 9.7 The ring-opening reaction occurred exclusively on the Zr–N bond, because no deuterium incorporation was found in the Et group of products 4 in the catalytic reaction when the reaction mixture was quenched with MeOD. As expected, this catalytic reaction did not proceed with MeMgBr. When higher magnesium alkyls, namely, n-PrMgBr and n-BuMgCl, were used, the formation of addition products was not observed from the reaction with imine 3a, indicating no coupling reaction between the zirconocene-substituted olefin complex and the imine.
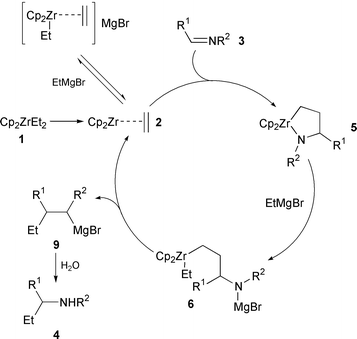 | ||
| Scheme 1 | ||
Notes and references
- T. Takahashi, T. Seki, Y. Nitto and M. Saburi, 37th Symposium on Organometallic Chemistry, Japan, Osaka, 1990, 172 Search PubMed; T. Takahashi, T. Seki, M. Saburi and E. Negishi, XIVth International Conference on Organometallic Chemistry, Detroit, MI, 1990, B17 Search PubMed; T. Takahashi, T. Seki, Y. Nitto, M. Saburi, C. J. Rousset and E. Negishi, J. Am. Chem. Soc., 1991, 113, 6266 Search PubMed.
- T. Takahashi, N. Suzuki, M. Hasegawa, Y. Nitto, M. Kageyama and M. Saburi, 38th Symposium on Organometallic Chemistry, Japan, Kyoto, 1991, 232. Search PubMedSee also T. Takahashi, N. Suzuki, M. Kageyama, Y. Nitto, M. Saburi and E. Negishi, Chem. Lett., 1991, 1579 Search PubMed For the structure of zirconium–olefin ate complex, see H. Lee, T. Hascall, P. J. Desrosiers and G. Parkin, J. Am. Chem. Soc., 1998, 120, 5830 CAS.
- For related catalytic reactions involving zirconocene, see also K. S. Knight and R. M. Waymouth, J. Am. Chem. Soc., 1991, 113, 6268 CrossRef CAS; A. H. Hoveyda and Z. Xu, J. Am. Chem. Soc., 1991, 113, 5079 CrossRef CAS; D. P. Lewis, P. M. Muller and R. J. Whitby, Tetrahedron Lett., 1991, 32, 6797 CrossRef CAS.
- U. M. Dzhemilev, O. S. Vostrikova and R. M. Sultanov, Izv. Akad. Nauk SSSR, Ser. Khim., 1983, 218 Search PubMed; U. M. Dzhemilev and O. S. Vostrikova, J. Organomet. Chem., 1985, 285, 43 CrossRef CAS.
- K. Kasai, M. Kotora, N. Suzuki and T. Takahashi, J. Chem. Soc., Chem. Commun., 1995, 109 RSC.
- T. Takahashi, M. Kotora and Z. Xi, J. Chem. Soc., Chem. Commun., 1995, 1503 RSC.
- Y. Zhang, J. Jiang and Y. Chen, Tetrahedron Lett., 1987, 28, 3815 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: reaction procedures and NMR data. See http://www.rsc.org/suppdata/cc/b0/b007456j/ |
| This journal is © The Royal Society of Chemistry 2001 |
