Synthesis of hyperbranched polymers from commercially available A2 and BB′2 type monomers
Chao Gao and Deyue Yan*
College of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, Peoples Republic of China.. E-mail: dyyan@mail.sjtu.edu.cn
First published on 19th December 2000
Abstract
This work describes a new strategy for the preparation of hyperbranched polymers from commercially available A2 and BB′2 type monomers without any catalysts; the rapid reaction of A groups with B groups results in the formation of dimers that can be regarded as a new sort of AB′2 type monomer; further polymerization of AB′2 gives hyperbranched polymers; through this approach a new kind of water-soluble hyperbranched polysulfone-amine was synthesized by polyaddition of N-methylpropane-1,3-diamine (BB′2) to divinyl sulfone (A2).
Hyperbranched polymers have received attention for about 10 years due to their unique chemical and physical properties.1–9 It is known that hyperbranched polymers are prepared mainly by polycondensation of ABx type monomers.10–14 Most ABx type monomers are unavailable commercially. A significant approach to hyperbranched polymers, therefore, would be the polymerization of commercially available difunctional and trifunctional monomers. Unfortunately, the polycondensation of A2 and B3 type monomers usually leads to the formation of a three-dimensional network.1 Interestingly, Kakimoto and co-workers15 reported that hyperbranched aromatic polyamides were synthesized by polycondensation of diamines (A2) with trimesic acid† (B3) at low monomer concentration in the presence of special catalysts. The hyperbranched polymers were accessible only when the feed monomer ratio was 1∶1. Gelation occurred within 10–20 min when the feed ratio of A2 to B3 was 3∶2.15,16
This work found that gelation can be avoided at different feed ratios if A2 monomers and BB′2 (instead of B3) monomers are used. Both B and B′ can react with A, and B is more active than B′. The difference in reactivity may be due to the distinction in chemical environment or chemical structure of the two functional groups. If the reaction between B and A is much faster than that between B′ and A, the predominant intermediate formed at the initial stage of the polymerization is the dimer (AA–BB′2), which can be regarded as a new kind of AB′2 type monomer. Further polymerization of AB′2 gives a hyperbranched polymer. The approach for synthesis of hyperbranched polymers from two types of reactive monomers is essentially different from that invented by Davis and co-workers in which the new functional group A is formed by reacting a B3 monomer with a reagent.17
To test the aforementioned concept, two kinds of monomers were selected as the raw materials (Scheme 1). Monomer 1 is attractive due to its strong electron-withdrawing group (SO2) linking double bonds. The sulfone group may guarantee the high reactivity of vinyl groups in the nucleophilic addition, which results in fast reaction without catalyst.18 The structure of 2 is interesting since it contains an active hydrogen atom in the secondary-amino group and two active hydrogen atoms in the primary-amino group. The former is more active than the latter in the nucleophilic addition due to the effect of the electron-donating group attached to the secondary-amino group.19,20 At the start of the polymerization, secondary-amino groups of 2 react rapidly with vinyl groups of 1 to afford 3. Species 3 is now an AB′2 monomer with one double bond and two active hydrogen atoms. Further polymerization of 3 leads to higher species and finally to a hyperbranched polymer.
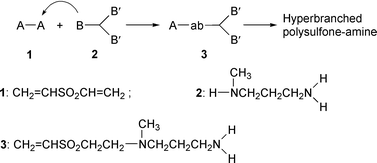 | ||
| Scheme 1 Polymerization mechanism of A2 and BB′2 monomers. | ||
FTIR has been used to investigate the reaction process. For the polyaddition of 1 to 2 with a mole ratio of 1∶1 (Fig. 1), the area integration of the absorption band from 1625 to 1602 cm−1 (assigned to double bonds) decreases to approximately a half of feed 1 during the initial 12 s. However, little change can be observed for the absorption bands from 3240 to 3450 cm−1 (assigned to primary-amino groups; the secondary-amino groups can not be observed in the IR spectrum). Then, the peaks of primary-amino groups and double bonds gradually decrease with the reaction. At about 5 h, the peak owing to the double bonds disappears completely, while that of the primary-amino groups is still observed (Fig. 2). These data fully support the polymerization mechanism shown in Scheme 1. Close monitoring of this polymerization by HPLC further demonstrates the reaction is initially very fast. The corresponding peak of monomer 1 vanished after about 10 s. Moreover, in the mass spectrum of the reaction mixture at the initial reaction stage, the molecular ion peak of the AB′2 monomer (M = 206.3) is observed at m/z = 207.1 (M + 1), and the fragment ion peak (M = 190.3) for AB′2 losing its primary-amino group is found at m/z = 190.1. These data suggest that dimer 3 is indeed generated by the reaction of the secondary-amino groups with vinyl groups.
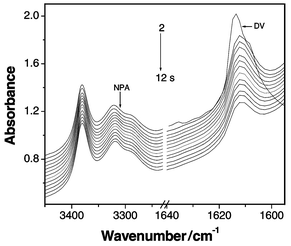 | ||
| Fig. 1 In situ FTIR spectra of the reaction mixture of dinvinyl sulfone (DV) and N-methylpropane-1,3-diamine (NPA) with the feed ratio of 1∶1 in chloroform within initial 12 s. | ||
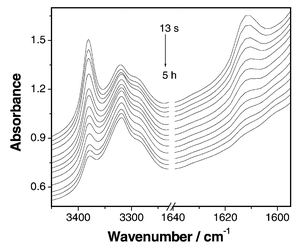 | ||
| Fig. 2 In situ FTIR spectra of the reaction mixture of dinvinyl sulfone and N-methylpropane-1,3-diamine with the feed ratio of 1∶1 in chloroform from 13 s to 5 h. | ||
When the initial mole ratio of A2 to BB′2 is equal to 1, the hyperbranched polymer was synthesized through the following procedure (P1 in Table 1): Under vigorous stirring and nitrogen atmosphere, divinyl sulfone (3.5445 g, 30 mmol) was added to a solution of N-methylpropane-1,3-diamine (2.6445 g, 30 mmol) in 15 ml of chloroform. The reaction mixture was kept at 40 °C for 96 h. Precipitation of the reaction mixture with 500 ml of MeOH and 8 M aqueous HCl (10 ml) gave the white hyperbranched polymer in 90% yield, which has a molecular weight, Mn, of 18530 (vapour-pressure osmometer) and a glass transition temperature of −22.8 °C (measured on a PE Pyris-1 DSC thermal analyzer under nitrogen at a heating rate of 20 °C min−1 from −80 to 200 °C). The resulting polymer shows good solubility in water and polar organic solvents such as DMF, NMP and DMSO, which provides further support for the polymerization mechanism without network formation. A combination of IR, 1H and 13C NMR spectra confirmed the structure of the hyperbranched polymer. For hyperbranched polymers, the degree of branching (DB)21 is calculated from the ratio of branched units (Nb) and terminal units (Nt) to the total units (branched units, terminal units and linear units). In this work, the branched unit is the tertiary-amino group, the terminal unit is the primary-amino group, and the linear unit (Nl) is the secondary-amino group. In the 1H NMR spectrum, the peaks of Nb, Nl and Nt were found at 3.58, 3.31 and 3.19 ppm, respectively. Additionally, a tertiary-amino group is also formed during the reaction of the A group and B group. Since there is a methyl group (CH3) attached to the tertiary nitrogen, the peaks of the formed tertiary-amino group (NB) are different from that of the branched tertiary-amino group in the 1H NMR spectrum. The peak of the methylene group (CH2) attached to NB was found at 3.75 ppm, and that of the methyl group at 2.9 ppm. Therefore, NB has no influence on the calculation of DB. The DB calculated from the integration ratio of corresponding peaks (Nb, Nl and Nt) is summarized in Table 1.
| Sample | Ratioa | Solvent | Time/h | Yield (%) | DB (%) | ηinhb | Mn |
|---|---|---|---|---|---|---|---|
| a The initial mole ratio of divinyl sulfone to N-methylpropane-1,3-diamine.b The inherent viscosity (dL g−1) of the resulting polymer measured at a concentration of 0.5 g dL−1 in water solution at 25 °C. | |||||||
| P1 | 1∶1 | CHCl3 | 96 | 90 | 58.7 | 0.17 | 18530 |
| P2 | 3∶2 | CHCl3 | 120 | 94 | 71.8 | 0.15 | 15385 |
| P3 | 1∶1 | NMP | 96 | 92 | 57.4 | 0.18 | 19860 |
| P4 | 3∶2 | NMP | 120 | 95 | 73.5 | 0.16 | 17110 |
When the feed ratio is equal to 3∶2, it is more difficult to avoid
gelation for the polycondensation systems of A2 and
B3 monomers.13,19 In this
article, no gelation occurred during the polymerization with the 3∶2
ratio in organic solvent. The end-capped polymer with benzoyl chloride is
soluble in chloroform and other polar solvents such as DMF, NMP, and DMSO.
Examination of the FTIR and HPLC showed the expected reaction mechanism as
given in Scheme 1. For the resulting
hyperbranched polymer with 3∶2-feed ratio, the terminal units should
contain vinyl groups and primary-amino groups. The peaks of the vinyl
groups were observed at 6.4 (CH2![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) ) and 6.8 (CH
) and 6.8 (CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) )
ppm in the 1H NMR spectrum.
)
ppm in the 1H NMR spectrum.
In conclusion, a novel approach for synthesis of hyperbranched polymers was presented. Water soluble hyperbranched polysulfoneamines were prepared by polyaddition of N-methylpropane-1,3-diamine (BB′2) to divinyl sulfone (A2). It is likely that the simplicity of this process and the generality of the method will make this new approach attractive for the synthesis of larger scale hyperbranched polymer materials.
This work was supported by the National Natural Science Foundation of China (No. 29974017) and the Gore & Associates Inc. of U. S. A.
Notes and references
- P. J. Flory, J. Am. Chem. Soc., 1952, 74, 2718 CrossRef CAS.
- Y. H. Kim, J. Am. Chem. Soc., 1992, 114, 4947 CrossRef CAS.
- J. M. J. Fréchet, Science, 1994, 263, 1710 CrossRef CAS.
- B. Voit, J. Polym. Sci., Polym. Chem., 2000, 38, 2505 Search PubMed.
- J. M. J. Fréchet, C. J. Hawker, I. Gitsov and J. W. Leon, J. Macromol. Sci.-Pure Appl. Chem., 1996, A33, 1399 Search PubMed.
- E. Malmströn and A. Hult, J. Macromol. Sci.-Rev. Macromol. Chem. Phys., 1997, C37, 555 Search PubMed.
- Y. H. Kim, J. Polym. Sci., Polym. Chem., 1998, 36, 1685 Search PubMed.
- T. Emrick, H. T. Chang and J. M. J. Fréchet, Macromolecules, 1999, 32, 6380 CrossRef CAS.
- J. M. J. Fréchet, H. Masahiro, I. Gitsov, S. Aoshima, M. R. Leduc and R. B. Grubbs, Science, 1995, 269, 1080 CrossRef CAS.
- Y. H. Kim and O. W. Webster, Macromolecules, 1992, 25, 5561 CrossRef CAS.
- V. Percec and M. Kawasumi, Macromolecules, 1992, 25, 3843 CrossRef CAS.
- T. M. Miller, T. X. Neenan, E. W. Kwock and S. M. Stein, J. Am. Chem. Soc., 1993, 115, 356 CrossRef CAS.
- D. H. Bolton and K. L. Wooley, Macromolecules, 1997, 30, 1890 CrossRef CAS.
- G. Yang, M. Jikei and M. Kakimoto, Macromolecules, 1999, 32, 2215 CrossRef CAS.
- M. Jikei, S. H. Chon, M. Kakimoto, S. Kawauchi, T. Imase and J. Watanebe, Macromolecules, 1999, 32, 2061 CrossRef CAS.
- S. M. Aharoni, Macromolecules, 1991, 24, 235 CrossRef CAS.
- N. J. Davis and S. P. Rannard, PCT Int. Appl. WO 9850453..
- K. Toda and T. Toda, Bull. Chem. Soc. Jpn., 1968, 41, 2519.
- J. R. Mohfig, S. S. Fu, R. W. King, R. Warnet and G. Gustafson, J. Am. Chem. Soc., 1990, 112, 3665 CrossRef.
- S. T. McDowell and C. J. M. Stirling, J. Chem. Soc. (B), 1967, 343, 348 RSC.
- C. J. Hawker, R. Lee and J. M. J. Fréchet, J. Am. Chem. Soc., 1991, 113, 4583 CrossRef CAS.
Footnote |
| † The IUPAC name for trimesic acid is benzene-1,3,5-tricarboxylic acid. |
| This journal is © The Royal Society of Chemistry 2001 |
