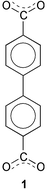
The first pillared three-dimensional structure constructed by carboxylate ligands bridging heterometallic trilayers
Long Pan, Bruce S. Finkel, Xiaoying Huang and Jing Li*
Department of Chemistry, Rutgers University, Camden, NJ 08102, USA.. E-mail: jingli@crab.rutgers.edu
First published on 19th December 2000
Abstract
Reaction of an aqueous solution of Na2bpdc (H2bpdc = biphenyldicarboxylic acid) with an organic solution of Co(NO3)2via a diffusion route leads to a pillared three-dimensional structure in which the heterometallic sodium(I)–cobalt(II) trilayers are connected by carboxylate groups of the pillared ligand bpdc2−.
One of the challenges in crystal engineering is to predict the crystal packing from molecular structures. A reasonable approach to build three-dimensional structures based on layered structural motifs is to control the packing of layers by organic pillars of changeable length and/or type. This approach has been demonstrated to be effective towards assembly of both non-covalent and covalent pillared networks.1 Pillared structures are potentially important for applications in absorption, separation and catalysis.2 A variety of pillar-layered structures including positively and negatively charged or neutral layers have been synthesized.3 Organically templated molybdenum oxides MOXI-1, -8, -24, -37 and indium phosphate (InPO) involve nitrogen donor pillars that connect to oxide layers.4 Metal phosphonates of the type Mz+4/z(O3P–R –PO3) use phosphonate pillars O3P–R–PO3 where R is an alkyl or aryl group.5 However, only a few pillared structures are built upon ligands containing carboxylate groups. Limited examples include exo-bicarboxylate in bilayer6 and isonicotinate (with a carboxylate group and a pyridine nitrogen) in three-dimensional pillared structures.7 In this contribution, we report a three-dimensional structure consisting of sodium(I)–cobalt(II) heterometallic trilayers and biphenylenecarboxylate pillars, 1. It is interesting to note that this structure represents the first example of carboxylate pillared structures containing heterometallic layers.
The title compound was synthesized by mixing organic and aqueous solutions via slow diffusion techniques. A DMF solution of Co(NO3)2 (0.1 M, 1 mL) was carefully layered on an aqueous solution of Na2bpdc (0.1 M, 1 mL), through a 2 mL mixed (buffer) solution of EtOH–water (2∶3). Pink block crystals of Na2Co(bpdc)2(H2O) 2, suitable for X-ray diffraction analysis,† appeared after several days. Single crystal X-ray analysis revealed that each sodium ion has a distorted triangular bipyramidal (tbp) coordination. The apical positions of the tbp are occupied by two carboxylate oxygen atoms (O1, O2) from two bpdc2−, while the equatorial positions are occupied by a η2-oxygen atom (O5) of a water molecule and two carboxylate oxygen atoms (O3, O4) from another two bpdc2−. Each sodium metal is connected with four adjacent sodium atoms located in the same plane through four carboxylate groups via anti–anti mode, leading to a two-dimensional grid-like metal network with Na⋯Na distances of 6.083 Å (along the b-axis) and 6.809 Å (along the c-axis). Two of these topologically identical Na planes are interconnected by Co. The octahedrally coordinated cobalt ion is located at the inversion center as a node, connected to four adjacent sodium ions through four O atoms at equatorial positions, two of which are trans η2-oxygen atoms (O5, O5i) of water molecules, and the other two, trans carboxylate η2-oxygen (O1, O1i). The remaining two (axial) positions around Co(II) are occupied by terminal water molecules (O6, O6i). This arrangement results in a heterometallic metal slab consisting of a Na(I)–Co(II)–Na(I) trilayer running parallel to the bc plane, with the edge-shared sodium(I) and cobalt(II) separated by 3.397(2) Å. A view of the metal slab is illustrated in Fig. 1. This metal trilayer is quite different from that of a pillared zinc biphenylylenebis(phosphonate) Zn2(O3PC12H8PO3) ·2H2O,8 in which not only the metal atoms are connected to five neighboring metals by four PO3 groups, but also they form a monometallic single layer.
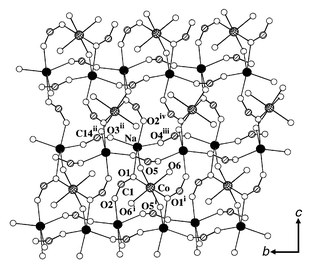 | ||
| Fig. 1 View of 2 along the crystallographic a-axis. Solid and cross-shaded circles are Na and Co, and open and shaded circles are O and C atoms, respectively. Selected bond lengths (Å) and angles (°): Na–Co 3.397(2), Co–O(1) 2.042(3), Co–O(6) 2.044(3), Co–O5 2.210(3), Na–O(1) 2.530(4), Na–O(5) 2.509(3), Na–O(2)iv 2.487(4), Na–O(4)iii 2.271(4), Na–O(3)ii 2.238(5); Na–Co–Nai 180, Na–O(5)–Co 91.88(11), Na–O(1)–Co 95.36(14). Symmetry codes: i −x + 1/2, −y + 1/2, −z−1; ii −x, y, −z + 1/2; iii: −x, y + 1, −z + 1/2; iv: x, −y, z − 1/2. | ||
The unique heterometallic trilayers in 2 are cross-linked by bpdc2− ligands to form a pillared three-dimensional structure (Fig. 2). Two CO2− groups of each bicarboxylate attach the adjacent metal layers as a stud. Each group adopts a different coordination mode. One binds a metal layer through its two oxygen atoms to two sodium ions in a bidentate anti–anti mode. The other, at the opposite end of the bpdc2−, forms tridentate bonds with the neighboring layer through its two oxygen atoms, coordinating to two sodium ions (in an anti–anti mode) and to one cobalt ion (with the η2-oxygen). The twisting of the bpdc2− ligand is a result of orientation adjustment to achieve a stable coordination geometry for both carboxylate and the metal ions from the layers above and below the pillar, and the skew–skew mode of the CO2− groups. The width of the heterometallic trilayer is 3.75 Å. The distance between the two neighboring trilayers is 11.5 Å. The shortest average distance between neighboring benzene rings is 3.75 Å, falling in the range of π–π interactions. This non-covalent interaction may stabilize the pillared structure. However, the closely packed biphenylene groups in the title compound are too close to accommodate guest solvent molecules. The connectivity of bpdc2− (μ5) in this heterometallic structure cannot be obtained by mixing different ratios of aqueous solutions of Na2bpdc (0.1 M) and Co(NO3) (0.1 M), (VNa2bpdc/VCo(NO3) 2 = 1∶1, 1∶5, 1∶10, 10∶1, 5∶1; V = volume). The product from these reactions is a one-dimensional structure with a single Co ion center, 1∞ [Co(bpdc)(H2O)2],9 which was confirmed by powder XRD. The one-dimensional chain in this structure was formed by connecting six-coordinated Co ions through the four μ-H2O, and two trans μ-bpdc.
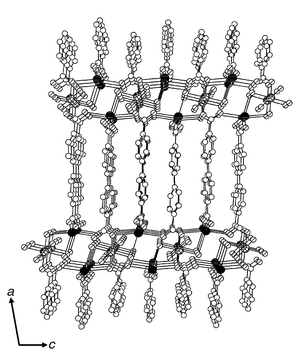 | ||
| Fig. 2 Perspective view of 2 down the b-axis, The same labeling scheme as in Fig. 1 is used. The width of heterometallic trilayer is 3.75 Å and the interlayer distance is 11.5 Å. | ||
The present work has shown that heterometallic pillared structures can be effectively synthesized using a bicarboxylate as a pillar molecule. Furthermore, it is likely that other transition metal elements with similar coordination habits and pillars of different length may replace Na/Co and bpdc2−, respectively, to form other related pillared structures. Suitable choices of ligands may also lead to porous, 3D pillared networks.
Acknowledgements
This work is generally supported by the National Science Foundation (Grant DMR-9553066).Notes and references
- S. C. Zimmerman, Science, 1997, 276, 543 CrossRef CAS; V. A. Russell, C. C. Evans, W. Li and M. D. Ward, Science, 1997, 276, 575 CrossRef CAS; M. Kondo, T. Okubo, A. Asami, S. Noro, T. Yoshitomi, S. Kitagawa, T. Ishii, H. Matsuzaka and K. Seki, Angew. Chem., Int. Ed., 1999, 38, 140 CrossRef CAS.
- Pillared Layered Structures: Current Trends and Applications, ed. I. V. Mitchell, Elsevier, London, 1990. Search PubMed.
- S. P. Newman and W. Jones, New J. Chem., 1998, 22, 105 RSC; T. J. Pinnavaia, Science, 1983, 220, 365 CrossRef CAS; G. K. H. Shimizu, G. D. Enright, C. I. Ratcliffe and J. A. Ripmeester, Chem. Commun., 1999, 461 RSC; G. Alberti, U. Costantino, C. Dionigi, S. Murciamascaros and R. Vivani, Supramol. Chem., 1995, 6, 29 Search PubMed.
- P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2639 CAS; D. E. Hagrman and J. Zubieta, J. Solid State Chem., 2000, 152, 141 CrossRef CAS; A. M. Chippindale, S. J. Brech and A. R. Cowley, Chem. Mater., 1996, 8, 2259 CrossRef CAS.
- A. Clearfield, Chem. Mater., 1998, 10, 2259 CrossRef; D. Medoukali, P. H. Mutin and A. Vioux, J. Mater. Chem., 1999, 9, 2553 RSC.
- L. Pan, X. Y. Huang, J. Li, Y. G. Wu and N. W. Zheng, Angew. Chem., Int. Ed., 2000, 39, 527 CrossRef CAS.
- O. R. Evans and W. Lin, Inorg. Chem., 2000, 2189 CrossRef CAS.
- B. Zhang, D. M. Poojary and A. Clearfield, Inorg. Chem., 1998, 37, 1844 CrossRef CAS.
- L. Pan, N. Ching, X.-Y. Huang and J. Li, Inorg. Chem., 2000, 39, 5333 CrossRef CAS.
Footnote |
| † Crystal data for 2:
Na2CoC28H24O12, M =
657.38, monoclinic, space group C2/c (no.15), a
= 3.746(6), b = 6.083(1), c = 13.363(3) Å,
Z = 4, V = 2500.0(8) Å3,
Dc = 1.747 g cm−3, crystal size 0.10
× 0.06 × 0.04 mm, μ(Mo-Kα) = 0.795
mm−1. The intensity data were collected with an
Enraf-Nonius CAD4 diffractometer using graphite-monochromated
Mo-Kα radiation (λ = 0.71073 Å). 196
unique reflections of which 1097 were measured;
R1[I >
2σ(I)] = 0.063, wR2
(all data) = 0.0668, GOF = 0.951. The structure were solved by direct
methods (SHELXS-86) and refined by full-matrix least-squared methods
(SHELXL-97). All non-hydrogen atoms were refined anisotropically. CCDC 182/1862. See http://www.rsc.org/suppdata/cc/b0/b007344j/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2001 |