Supercritical fluid mixing: preparation of thermally sensitive polymer composites containing bioactive materials†
Steven M. Howdle*a, Michael S. Watsona, Martin J. Whitakerb, Vladimir K. Popovc, Martyn C. Daviesb, Frederick S. Mandeld, J. Don Wangd and Kevin M. Shakesheffb
aSchool of Chemistry, University of Nottingham, Nottingham, UK NG7 2RD. E-mail: steve.howdle@nottingham.ac.uk
bSchool of Pharmaceutical Sciences, University of Nottingham, Nottingham, UK NG7 2RD
cInstitute of Laser and Information Technology, LaserChem Group, Russian Academy of Sciences, 2 Pionerskaya Str., Troitsk, 142092, Russia
dFerro Corporation, 7500 E. Pleasant Valley. Rd., Independence, OH 44131, USA
First published on 15th December 2000
Abstract
We report the use of supercritical carbon dioxide (scCO2) to create a diverse range of polymeric composites incorporating thermal and solvent labile guest materials such as proteins; no additional co-solvents are required; the entire process can be carried out at near ambient conditions; polymer morphology is controllable; high loadings of guest species can be achieved and the protein function is retained.
Tissue engineering scaffolds and controlled drug delivery devices are polymeric composites designed to release precise amounts of guest species, e.g. growth factors and biotechnology drugs, at rates matching the physiological need of the tissue.1 The challenge is to incorporate such biologically active guest species, without loss or change of activity, into a polymer host. There are well documented problems in maintaining protein conformation and activity under conventional processing methods due to either the presence of an organic/aqueous solvent interface (e.g. double emulsion particle formation), elevated temperatures (e.g. polymer melt processing), or mechanical agitation of solutions.2 A further challenge in producing these composites is to control the morphology of the composite, e.g. to generate a pore size distribution that promotes cell infiltration or a controlled porosity to influence polymer degradation and controlled release characteristics.
Here, we report a supercritical fluid mixing technology that overcomes both of these limitations in one processing step. The combination of gas-like and liquid-like properties makes scCO2 a unique polymer-processing medium.3,4 scCO2 has long been proposed as the ideal medium for preparation of polymer based materials containing bioactive species, primarily because no solvent residues would remain in the final product. Progress has been slow because scCO2 is generally a poor solvent for polar guest species5 and for polymers.6 and this has resulted in extensive use of scCO2 as an anti solvent.7,8
However, scCO2 does interact with a broad range of amorphous polymers leading to depression of the glass transition temperature (Tg).4 Under these conditions the polymer is plasticised, substantially lowering the viscosity and allowing efficient incorporation of insoluble guest particles into the polymer (see Contents Page illustration). Briefly, powdered samples of polymer and bioactive guest are placed in the autoclave and scCO2 added. Neither the guest, nor the polymer, dissolves in scCO2. The precise conditions of temperature and pressure required to plasticise the polymer are determined by the composition of the polymer. For the biodegradable polymers poly(DL-lactide) (PLA), poly(lactide-co-glycolide) (PLGA) and polycaprolactone (PCL), near ambient temperature (35 °C) and modest pressures (200 atm) are sufficient. Efficient agitation of the scCO2 swollen polymer leads to homogeneous distribution of the guest particles throughout the polymer matrix. The vessel is then depressurised to produce either monolithic samples or encapsulated particles. For monoliths, the composite material is formed inside the autoclave. For particles, the entire contents of the vessel are forced out under pressure through a computer controlled orifice. Depressurisation is rapid, and is controlled by the action of a flush valve. The encapsulated particles are retrieved from a cyclone collector and the CO2 may be repressurised and recycled. The methodology is based on the technique of particle generation from supercritical suspension (PGSS) and was initially developed for the preparation of powder coatings for paint applications, with the aim of decreasing processing temperatures.9 The whole process can be performed in <1 h at near ambient temperatures on a gram to multi kg scale in a single processing step. No solvent residues remain after processing and a wide range of loadings (up to 70% by weight), morphologies, and porosities are accessible.
To demonstrate the principle we have prepared porous composites of PLGA (75% DL-lactide∶25% glycolide, Mw = 26 kDa, intrinsic viscosity = 0.6) incorporating a high loading of calcium hydroxyapatite (HA). Such materials are required for bone regeneration applications (Fig. 1A, B). A PLGA composite incorporating the enzyme catalase at 50% w/w loading is also shown (Fig. 1C) The catalase particle morphology is totally unchanged after processing, the protein is intimately mixed with the biodegradable polymer, and porosity in the polymer phase is clearly evident.
![SEMs of composites. (A and B) Images of internal fracture surface of
composite of HA (40 wt%) and PLGA (60 wt%). At low magnification the
distribution of HA and the porosity is evident, at higher magnification the
intimate mixing of HA and PLGA is observed. (C) Catalase (50 wt%)
incorporated into a PLGA matrix (50%). Micron-scale pores in the polymer
and the distinctive protein particle morphology are evident. (D)
High-surface area microparticle composite [fluorescein (sodium salt) (8
wt%) and PCL (92 wt%)] produced by direct atomisation.](/image/article/2001/CC/b008188o/b008188o-f2.gif) | ||
| Fig. 1 SEMs of composites. (A and B) Images of internal fracture surface of composite of HA (40 wt%) and PLGA (60 wt%). At low magnification the distribution of HA and the porosity is evident, at higher magnification the intimate mixing of HA and PLGA is observed. (C) Catalase (50 wt%) incorporated into a PLGA matrix (50%). Micron-scale pores in the polymer and the distinctive protein particle morphology are evident. (D) High-surface area microparticle composite [fluorescein (sodium salt) (8 wt%) and PCL (92 wt%)] produced by direct atomisation. | ||
The ability to plasticise such polymers at close to 37 °C and the inherently low solubility of proteins in scCO2 provides a key processing advantage; thermal and solvent labile species can be processed easily whilst preserving protein structure and function. We have demonstrated this for three enzymes; ribonuclease A, catalase and β-D-galactosidase. For ribonuclease A, a number of investigators have studied the activity after exposure to a wide range of solvents and environments using a standard assay.10 Using scCO2 (170 atm; 37 °C for 10 min), we have prepared composites of ribonuclease A (10 wt%) and PLA (Mw 30 kDa). The enzyme was then released to a Tris buffer at 37 °C for 5 min and analysed (see ESI†). The total concentration of protein released was verified by a Coomassie protein assay and the activity of the released protein was measured. The data presented in Fig. 2 demonstrate that the activity of the enzyme is preserved. Thus scCO2 behaves in a similar manner to conventional anhydrous solvents, with the advantage that no solvent residues are introduced. Indeed, scCO2 processing even removes the conventional solvent residues that are almost always found in guest and polymer materials. Clinical use of such composites requires controlled release of the guest from the polymer host. Release of ribonuclease A from the PLA composites over a 70 day period revealed an initial burst release of <10% of the dose over the first 2 days followed by close to zero-order release kinetics until the delivery device was exhausted.
 | ||
| Fig. 2 Evidence of retention of ribonuclease A activity. Measured activities of the released enzyme (four grey-filled symbols) correlate well with calibration data (◆ symbols) and best fit line. | ||
We have also demonstrated that this scCO2 mixing technology leads to fine control of the morphology of polymer composites. Others have demonstrated that scCO2 can be used to create microcellular foams of amorphous polymers, and that control of the magnitude of pressure drop and the rate of depressurisation influence strongly the porosity and pore size.11,12 The data in Fig. 3A were recorded following a ‘slow’ (over a 2 h period), whereas Fig. 3B shows the effect of a ‘fast’ (2 min) depressurisation. SEM images show that the ‘slow’ material possesses a small population of large pores (diameter 100–500 μm) whereas the ‘fast’ material has higher porosity and the pore size distribution, within the resolution of SEM, is more heterogeneous. Mercury porosimetry reveals significant differences in the pore size distribution of small pores that are not visible in the SEM images. ‘Slow’ depressurisation produced micropores between 50 and 100 nm diameter and no pores in the 500 nm to 5 μm range. Conversely, ‘fast’ depressurisation produced no micropores in the 50–100 nm range, but many in the 500 nm to 5 μm range. Thus, supercritical mixing offers a mechanism of controlling both macro- and micro-porosity without the addition of porogens13 and via a one-step process. We have also observed such macro- and micro-porosity in protein loaded composites.
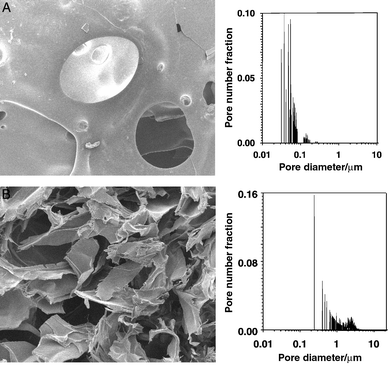 | ||
| Fig. 3 Controlling PLA pore structure. A, ‘slow’ depressurisation over a 2 h period. B, ‘fast’ depressurisation over a 2 min period. | ||
For many applications, the polymer/drug composite is required as micron-scale particles particularly for controlled release and lung or subcutaneous delivery systems.1 Our technique can also produce such micron-scale composite particles. After the mixing step, direct atomisation is achieved by releasing the pressure of the system across an orifice of known diameter, with particles forming as the CO2 expands rapidly. The image in Fig. 2D shows an example of the type of particle morphology generated when processing a model system. The composite particles have a high surface area and their size may be fine-tuned by control of the orifice diameter. Typically the mean diameters fall in a range between 24 and 50 μm, a range that has been shown to be suitable for lung delivery of low density particles.14
The novel supercritical mixing process described here requires neither the guest nor the polymer to dissolve in scCO2. The process is performed at near ambient temperature and conventional solvents are not required.15 Hence, it is especially applicable to thermally labile or solvent sensitive guest species and polymers. Most importantly we have demonstrated that protein functioning is retained for a range of enzymes after the processing. The technique is applicable to production of composites for any technology requiring thermally labile materials dispersed throughout a polymer host.
Acknowledgements
We thank the Royal Society and EPSRC for Fellowships (S. M. H. and K. M. S.) and the RFBR for support.Notes and references
- R. Langer, Nature, 1998, 392, 5 CAS.
- K. Fu, A. M. Klibanov and R. Langer, Nat. Biotechnol., 2000, 18, 24 CrossRef CAS.
- M. A. McHugh and V. J. Krukonis, Supercritical Fluid Extraction; Principles and Practice, Butterworth, London, 1994. Search PubMed.
- A. I. Cooper, J. Mater. Chem., 2000, 10, 207 RSC.
- A. J. Mesiano, E. J. Beckman and A. J. Russell, Chem. Rev., 1999, 99, 623 CrossRef CAS.
- F. Rindfleisch, T. P. DiNoia and M. A. McHugh, J. Phys. Chem., 1996, 100, 15581 CrossRef CAS.
- B. Subramaniam, R. A. Rajewski and K. Snavely, J. Pharm. Sci., 1997, 86, 885 CrossRef CAS.
- T. J. Young, K. P. Johnston, K. Mishima and H. Tanaka, J. Pharm. Sci., 1999, 88, 640 CrossRef CAS.
- F. S. Mandel, 5th Meeting on Supercritical Fluids, Nice, France, 1998, p. 69. Search PubMed.
- D. B. Volkin, A. Staubli, R. Langer and A. M. Klibanov, Biotechnol. Bioeng., 1991, 37, 843 CrossRef CAS.
- S. K. Goel and E. J. Beckman, Polym. Eng. Sci., 1994, 34, 1137 Search PubMed.
- D. J. Mooney, D. F. Baldwin, N. P. Suh, L. P. Vacanti and R. Langer, Biomaterials, 1996, 17, 1417 CrossRef CAS.
- M. H. Sheridan, L. D. Shea, M. C. Peters and D. J. Mooney, J. Controlled Release, 2000, 64, 91 CrossRef CAS.
- D. A. Edwards, J. Hanes, G. Caponetti, J. Hrkach, A. BenJebria, M. L. Eskew, J. Mintzes, D. Deaver, N. Lotan and R. Langer, Science, 1997, 276, 1868 CrossRef CAS.
- D. D. Hile, M. L. Amirpour, A. Akgerman and M. W. Pishko, J. Controlled Release, 2000, 66, 177 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: methods and measurement of ribonuclease A activity. See http://www.rsc.org/suppdata/cc/b0/b008188o/ |
| This journal is © The Royal Society of Chemistry 2001 |
