Fabrication of a novel polypyrrole/poly(methyl methacrylate) coaxial nanocable using mesoporous silica as a nanoreactor†
Jyongsik Jang*, Byungkwon Lim, Jinwoo Lee and Taeghwan Hyeon*
School of Chemical Engineering, Seoul National University, Seoul, 151-742, Korea.. E-mail: jsjang@plaza.snu.ac.kr and thyeon@plaza.snu.ac.kr
First published on 14th December 2000
Abstract
We report on the fabrication of a polypyrrole/poly(methyl methacrylate) coaxial nanocable through the sequential polymerization of methyl methacrylate and pyrrole monomers inside the channels of mesoporous SBA-15 silica, followed by the removal of the silica template.
There has been tremendous interest in the development of conducting structures with nanometer dimensions. These nanostructured conducting materials could find many attractive applications such as electromagnetic interference shielding, electrochromic devices, supercapacitors, polymeric electrodes and sensors.1 Recently, many nanotubules and nanowires of polymers, metals and carbons have been produced through template approaches with various membranes,2 layered inorganic solids,3 zeolites and mesoporous materials.4 Generally conducting polymers themselves, however, possess poor mechanical properties, and are very difficult to process. To overcome these drawbacks, much research effort has been focused on the development of processes to combine conducting polymers and polymer matrices with good tractability.5 Although many different conducting polymer composites have been developed, no nanostructured conducting-polymer/polymer composites have, as yet, been reported. Herein, we report the preparation of polypyrrole/poly(methyl methacrylate) coaxial nanocable using mesoporous silica as a template.‡
In the synthesis, mesoporous SBA-15 silica was utilized as a template. Mesoporous SBA-15 silica with regular hexagonal pores of 7.5 nm was prepared by the reported method.6 To synthesize tubular PMMA within the pores of SBA-15, methyl methacrylate (MMA) was incorporated into the pores of SBA-15 silica and MMA was subsequently polymerized. The amount of MMA was precisely controlled so that the polymerization could occur selectively on the inner surfaces of mesopores of SBA-15, resulting in a tubular structure of PMMA. Pyrrole monomer was polymerized using FeCl3 as an oxidant inside the tubular holes of the PMMA/SBA-15 composite, resulting in a PPy/PMMA/SBA-15 nanocomposite. The removal of SBA-15 silica template by etching using an aqueous HF solution produced a PPy/PMMA nanocomposite.
The pore size distribution (PSD) curves of SBA-15, PMMA/SBA-15 and PPy/PMMA/SBA-15 were derived from nitrogen adsorption isotherms and are presented in Fig. 1. The pore size of SBA-15 silica (7.5 nm) was decreased to 6.2 nm when the polymerization of MMA was conducted and the TEM image of PMMA incorporated SBA-15 exhibited a nearly hexagonal arrangement of pores, demonstrating the formation of tubular PMMA inside the mesopores of SBA-15 silica. The pore volumes of SBA-15, PMMA/SBA-15, and PMMA/PPy/SBA-15 are 0.63, 0.23, and 0.07 cm3 g−1, respectively. Thermogravimetric analysis (TGA) showed the weight ratio of SBA-15: PMMA∶PPy = 50∶27∶23 in the PPy/PMMA/SBA-15 composite. Elemental analysis of the PMMA/PPy nanocomposite revealed a 1∶1 weight ratio of PMMA and PPy, which matches well with the TGA data. The weight ratio can be also calculated from the change of pore volumes after considering the bulk densities (1.2 g cm−3 for PMMA and 1.5 g cm−3 for PPy) and the weight increases from the consecutive addition of two polymers. The ratio was calculated to be PMMA∶PPy = 1∶0.8. When the weight ratio was derived simply from the decrease in the pore sizes of the composites, the weight of PMMA is much lower (PMMA∶PPy = 0.4∶1). However, this discrepancy can be explained by the incorporation of PMMA into the complementary pores (micropores) of SBA-15 silica. Recently, Kruk et al. revealed that many micropores in the walls of SBA-15 silica interconnect primary mesopores.7 When polymerization of MMA occurs, PMMA might fill these micropores in addition to partial filling of the mesopores. Therefore, the actual amount of PMMA incorporated into SBA-15 is larger than the calculated amount of PMMA derived solely from the partial filling of mesopores.
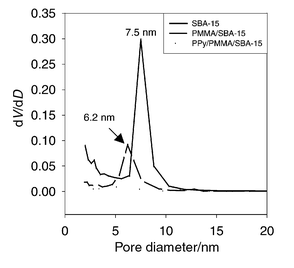 | ||
| Fig. 1 The pore size distribution curves of SBA-15, PMMA/SBA-15 and PPy/PMMA/SBA-15. The curves were obtained from the adsorption branch of the nitrogen isotherm calculated by the BJH method. The isotherms were collected at 77 K on a Micrometrics ASAP2010 Gas Adsorption Analyzer after degassing at 250 °C at 30 μTorr for 5 h. | ||
Small angle X-ray scattering (SAXS) patterns were obtained at various times during the synthesis. The diffraction pattern of SBA-15 showed typical hk0 reflections (100, 110 and 200) from the hexagonal arrangement of pores. The peak intensity in the SAXS of PMMA/SBA-15 and PPy/PMMA/SBA-15 decreased compared with that in SBA-15 silica, but the peak positions of the XRD patterns were nearly identical. These results show that the SBA-15 structure remained intact during the synthesis, demonstrating the usefulness of SBA-15 silica as a nanoreactor.
The PPy/PMMA nanocomposite was obtained by HF etching of the SBA-15 silica template. It has been reported that acid treatment does not decrease the electrical conductivity of PPy.8 The resulting polymer/polymer nanocomposite was dried at 100 °C under vacuum to obtain the dry powder which was analyzed by FTIR spectroscopy. The IR spectrum of the nanocomposite exhibited characteristic peaks from both PMMA and PPy. One very unusual observation is that there are two carbonyl peaks associated with PMMA. The curve-fitted spectra of the carbonyl region of the PPy/PMMA nanocomposite exhibited one major peak at 1727 cm−1 and a shoulder at 1700 cm−1 [Fig. 2(a)]. The carbonyl peak at 1700 cm−1 indicates hydrogen bonding interactions between PMMA carbonyl groups and PPy N–H groups. On the other hand, the peak at 1727 cm−1 arise from the free carbonyl groups of PMMA. Such a strong interaction between PPy and PMMA has not been reported before in the micrometer-sized PPy/PMMA composites. Judging from these data, it seems that the outer PMMA layer is intimately mixed with PPy at the nanometer-scale [Fig. 2(b)].
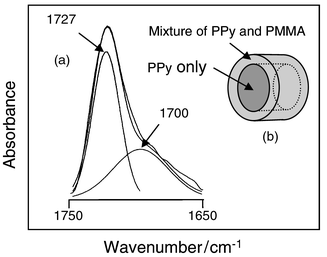 | ||
| Fig. 2 (a)Curve-fitted IR spectra of the PMMA carbonyl peak for the PPy/PMMA nanocomposite. (b) A schematic illustration of coaxial nanocable of PPy/PMMA. | ||
The PPy/PMMA nanocomposite could be easily molded by compression under 25 kN cm−2 at 220 °C for 10 min using a pelletizer. Interestingly, the AFM image of the compression-molded PPy/PMMA composite revealed highly oriented and unidirectional structures (Fig. 3). The well aligned structure was observed nearly all over the sample, which seems to result from template synthesis inside regular hexagonally arranged mesopores of SBA-15. The compression-molded PPy/PMMA nanocomposite exhibited an electrical conductivity of 1.7 S cm−1. Considering that the typical conductivity of chemically synthesized PPy lies in the range 1–10 S cm−1, the conductivity of our PPy/PMMA composite seems to be low. However, when we consider that the PPy wires in the PPy/PMMA nanocomposite are surrounded by insulating PMMA tubules, limiting full exposure of the conducting surface of PPy, the conductivity is relatively high which might result from the good alignment of the PPy chains.
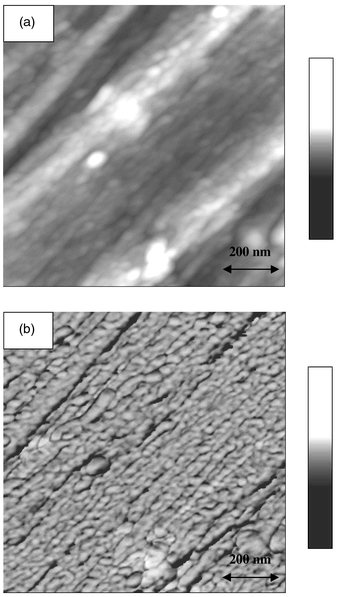 | ||
| Fig. 3 AFM images of PPy/PMMA nanocomposite: (a) height image and (b) phase image. The film was fabricated by compression-molding of the PPy/PMMA nanocomposite at 220 °C under 25 kN cm−2 for 10 min. The images were obtained with Nanoscope IIIa Dimension 3100 SPM (Digital Instruments) in tapping mode. | ||
Acknowledgements
We are grateful to the Brain Korea 21 Program supported by the Korean Ministry of Education for the financial support.Notes and references
- P. Kathirgamanathan, Adv. Mater., 1993, 5, 281 CAS; P. K. Shen, H. T. Huang and A. C. C. Tseung, J. Electrochem. Soc., 1992, 139, 1840 CAS; L. Diederich, E. Barborini, P. Piseri, A. Podesta, P. Milani, A. Schneuwly and R. Gallay, Appl. Phys. Lett., 1999, 75, 2662 CrossRef CAS; D. K. Moon, A. B. Padias, H. K. Hall, T. Huntoon and P. D. Calvert, Macromolecules, 1995, 28, 6205 CrossRef CAS; J. Kong, N. R. Franklin, C. W. Zhou, M. G. Chapline, S. Peng, K. J. Cho and H. J. Dai, Science, 2000, 287, 622 CrossRef CAS.
- C. R. Martin, Science, 1994, 266, 1961 CrossRef CAS; C. R. Martin, Acc. Chem. Res., 1995, 28, 61 CrossRef CAS; G. Che, B. B. Lakshmi, E. R. Fisher and C. R. Martin, Nature, 1998, 393, 346 CrossRef CAS; V. M. Cepak and C. R. Martin, Chem. Mater., 1999, 11, 1363 CrossRef CAS.
- M. G. Kanatzidis, L. M. Tonge, T. J. Marks, H. O. Marcy and C. R. Kannewurf, J. Am. Chem. Soc., 1987, 109, 3797 CrossRef CAS; M. G. Kanatzidis, C. G. Wu, H. O. Marcy and C. R. Kannewurf, J. Am. Chem. Soc., 1989, 111, 4139 CrossRef CAS.
- C. G. Wu and T. Bein, Science, 1994, 264, 1757 CrossRef CAS; C. G. Wu and T. Bein, Science, 1994, 266, 1013 CrossRef CAS; S. A. Johnson, E. S. Brigham, P. J. Ollivier and T. E. Mallouk, Chem. Mater., 1997, 9, 2448 CrossRef CAS; S. A. Johnson, D. Khushalani, N. Coombs, T. E. Mallouk and G. A. Ozin, J. Mater. Chem., 1998, 8, 13 RSC; J. Lee, S. Yoon, S. M. Oh, C.-H. Shin and T. Hyeon, Adv. Mater., 2000, 12, 359 CrossRef CAS.
- M. Omastova, J. Pavlinec, J. Pionteck, F. Simon and S. Kosina, Polymer, 1998, 39, 6559 CrossRef CAS; S. F. Lascelles and S. P. Armes, J. Mater. Chem., 1997, 7, 1339 RSC.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- M. Kruk, M. Jaroniec, C. Ko and R. Ryoo, Chem. Mater., 2000, 12, 1961 CrossRef CAS.
- M. Forsyth and V. T. Truong, Polymer, 1995, 36, 725 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: FTIR spectra, XRD patterns and TEM images. See http://www.rsc.org/suppdata/cc/b0/b006197m/ |
| ‡ Experimental details: SBA-15 was prepared by the reported method. 1 g of methyl methacrylate (MMA) was incorporated into the pores of SBA-15 silica (2 g) by heating for 5 h at 90 °C under reduced pressure. MMA was polymerized with 10 mg of benzoyl peroxide under an argon atmosphere at 70 °C for 2 days and then 120 °C for 2 h, followed by evacuating in a vacuum oven at the same temperature for 18 h. 0.4 g of pyrrole was loaded into the pores of the PMMA/SBA-15 (1.3 g) using the same conditions as in the MMA incorporation, and then polymerized with 20 mL of 0.81 M aqueous FeCl3 solution for 3 h. The resulting solid was retrieved by filtration, followed by drying under vacuum at room temperature for 12 h. To remove the silica template, the PPy/PMMA/SBA-15 composite was dispersed in an aqueous HF solution (48 wt%), and was stirred overnight. The PPy/PMMA nanocomposite was retrieved by vacuum filtration. To fabricate a thin film, the powder form of PPy/PMMA was compression-molded using a pelletizer under 25 kN cm2 at 220 °C for 10 min. |
| This journal is © The Royal Society of Chemistry 2001 |
