A mannose-6-phosphonate–cholesterylamine conjugate as a specific molecular adhesive linking cancer cells with vesicles
Véronique Barragan*a, Fredric M. Mengera, Kevin L. Carana, Carole Vidilb, Alain Morèreb and Jean-Louis Monterob
aDepartment of Chemistry, 1515 Pierce Drive, Emory University, Atlanta 30322, USA.. E-mail: menger@emory.edu
bLaboratoire de Chimie Biomoléculaire, UMR 5032, Université Montpellier II, Place Eugène Bataillon, 34095 Montpellier cedex 05, France.. E-mail: montero@univ-montp2.fr
First published on 14th December 2000
Abstract
A hydrolytically stable sugar phosphonate coupled to a steroid via a long and semi-rigid spacer (synthesized via a 12-step sequence) binds both to the mannose-6-phosphonate receptors of certain cancer cells and to the lipid bilayer of vesicles, thereby serving as a multivalent adhesive between cell and vesicle surfaces.
Mannose-6-phosphate plays a key role in the intracellular retention and secretion of lysosomal hydrolytic enzymes.1 Thus, this sugar residue inserted on the enzymes directs the transport of the newly synthesized enzymes from the Golgi apparatus to the lysosomes. Membrane-bound mannose-6-phosphate receptors assist in both the secretion and internalization of the lysosomal enzymes. It has been demonstrated that the population of mannose-6-phosphate receptors is increased abnormally in breast cancer cells as compared to those of benign breast disease.2 The receptors can, therefore, provide a handle with which to attack the cells.3 For example, drugs and vesicles endowed with mannose-6-phosphate moieties could, in principle, bind selectively to cancer cells owing to the enhanced presence of mannose-6-phosphate receptors. It is this potential vulnerability of the cancer cells which motivates the present paper. A recent paper describing vesicles containing phospholipids bearing a sugar-binding boronic acid that adhere to erythrocytes embodies a similar philosophy.4
Actually, the use of mannose-6-phosphate moieties to target bioactive molecules is not as attractive as it might seem. The problem lies in the susceptibility of sugar phosphates to hydrolysis by various phosphatase enzymes. To circumvent this problem, we have recently developed synthetic routes to two phosphonate analogs of mannose-6-phosphate (M6-P); one is isosteric with the phosphate moiety while the other is not.5 Both, however, are hydrolytically stable. Upon submitting the analogs for biological testing, it was found that the isostere (but not the non-isostere) binds to M6-P receptors as effectively as does M6-P itself.5
Encouraged by the results of our biological testing, we proceeded to construct a molecule (14) that could bind both to cancer cells and to vesicles and, in this manner, serve as an ‘adhesive’ between the living and chemical systems. Our interest in attachment to cancer cells arose from the widespread attention given to vesicles (also called ‘liposomes’) as drug delivery vehicles in cancer therapy.6
Vesicles are spherical objects composed of one or more lipid bilayer shells surrounding an aqueous interior. Their diameter ranges from 30 nm (‘small’) to 10–200 μm (‘giant’), and we have had considerable experience with both types.7 Often in our vesicle research we add cholesterol, owing to its high affinity for lipids bilayers and to its strengthening effect upon the vesicles. It was for these properties that a cholesterylamine unit was selected as the vesicle-adhering unit. All told, about a dozen steps were required to synthesize the cell/vesicle adhesive (Scheme 1). Reactions were generally run at the 50 mg level because scale-up often reduced the yields. Great attention to purification by chromatography was necessary throughout.
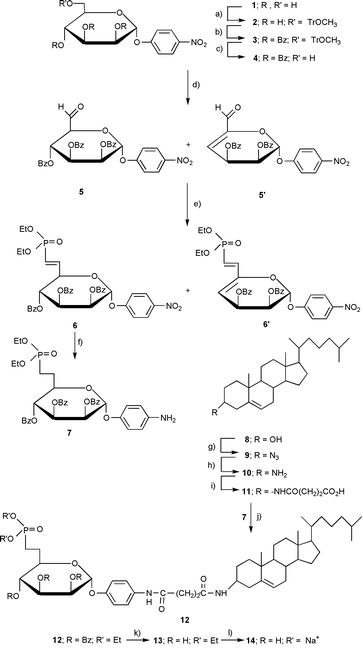 | ||
| Scheme 1 Reagents and conditions: (a) CH3OTRCl, pyridine, 94%; (b) BzCl, pyridine, 90%; (c) Ce(NH4)2NO3CN–H2O 95∶5, 80 °C, 90%; (d) Swern oxidation, (COCl)2, DIEA, DMSO, THF, 5: 37%, 5′: 63%; (e) Wittig–Horner, TEMDP, NaH, benzene, 6′: 40%; (f) H2 Pd/C, EtOH, 86%; (g) PPH3, HN3, DEAD, toluene, 65%; (h) LiAlH4, Et2O, 95%; (i) succinic anhydride, TEA, Et2O, 90%; (j) DCC/HOBT, DMAP, N-Et-morpholine, CH2Cl2, 80%; (k) MeOH–NH3, 4 °C, 90%; (l) Me3SiBr, pyridine, CH2Cl2 then Na cation exchange resin, 40%. | ||
4-Nitrophenyl-α-D-pyranoside 1 was used to initiate the synthesis (Scheme 1). Compound 1 was first selectively protected on the primary hydroxy with a 4-methoxytrityl group to give 2. The secondary hydroxys were then protected with benzoyl groups (2 to 3). Attempts to benzylate these hydroxys instead of benzoylating them led to degradation. Compound 3, fully protected, was selectively deprotected only at C-6 with the aid of a redox reaction involving ceric ammonium nitrate. The resulting primary alcohol 4 was then subjected to a Swern oxidation and converted into a product that gave two spots when developed with rhodanine. Both products showed the presence of an aldehyde proton in the NMR (one at 9.70 ppm and the other at 9.28 ppm in a ratio of 37 and 63 respectively). Purification of the mixture by chromatography gave the latter aldehyde whose NMR spectrum indicated the occurrence of a E1cb-type elimination of a benzoate during the Swern oxidation. In the course of the actual synthesis, however, the aldehyde mixture (5 and 5′) was used without separation in a Wittig–Horner reaction to produce two phosphonates (6 and 6′) in 25% and 40% yields, respectively. At this point, phosphonate 6 was isolated by chromatography and hydrogenated to 7, a reduction that both removed the unsaturation and converted the nitro group into an amino group. Meanwhile, in this convergent synthesis, chemistry was being performed on the steroidal portion of the molecule starting with cholesterol 8. Thus, cholesterol was transformed into α-cholesterylamine 10 using a literature procedure with only slight modification.8 We now had in hand the necessary two amines which were joined together via a succinoyl unit by first acetylating 10 with succinic anhydride to give 11. Carbodiimide coupling of 11 with 7 then gave 12 which needed only to be deprotected to produce the final compound.
Deprotection was accomplished by first debenzoylating 12 with methanolic ammonia. The phosphonate diester 13 was used to obtain the disodium phosphonate salt with the aid of trimethylsilylbromide; purification of 14 was performed by chromatography on silica gel. An overall yield of only 2.6% is indicative, in part, of the difficulties in preparing and purifying this large and bipolar molecule.
Microscopically visible giant vesicles, comprised of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), compound 14 or its phosphate counterpart,9 and 1-palmitoyl-2-oleoyl-3-phosphoglycerol (POPG) were prepared in Hepes solution (1 mM) by the hydration method,7 using ratios from 87∶5∶8 to 75∶17∶8, respectively. An aliquot of the vesicle suspension was added to a petri dish containing MCF-7 breast cancer cells (half-confluent) in Dulbecco’s phosphate buffer solution (DPBS, Sigma). The petri dish was gently swirled and placed under a light microscope. Giant vesicles adjacent to cancer cells were grasped with a micropipet under suction and slowly withdrawn from the cells. The majority of the giant vesicles containing 14 or its phosphate counterpart failed to release the cells but continued to stick to them. When such vesicles were pulled away from the cancer cells, either the cell (Fig. 1) or the vesicle (Fig. 2) was substantially distorted. Fig. 1 shows how a withdrawn vesicle retains its contact with the cancer cell via an elongated fiber originating from the cancer cell. Fig. 2 shows how a spherical giant vesicle, attached to a cancer cell via14, deforms into an ellipsoid upon application of a lateral force. Control runs, carried out with identical vesicles except that their bilayers possessed a non-adhesive steroid, the succinate derivative of cholesterylamine, failed to produce a similar effect in the majority of cases. These experiments suggest that 14 does indeed serve as a chemical adhesive.
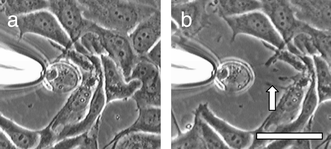 | ||
| Fig. 1 A giant vesicle containing the phosphate counterpart of 14 has attached itself to a cluster of cancer cells. (b) The vesicle is slowly pulled away from the cells with a micropipet. A fiber (arrow) forms between the vesicle and cells. Bar = 20 μm. | ||
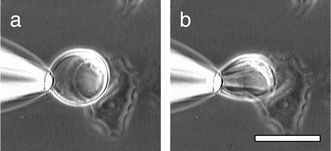 | ||
| Fig. 2 A giant vesicle containing adhesive 14 and attached to a cancer cell showing a sphere-to-ellipsoid distortion upon application of a lateral force. Bar = 20 μm. | ||
Submicroscopic vesicles (100 nm in diameter) were prepared by hydration of a film of POPC–14–POPG mixture with a 0.96 mM solution of a water-soluble fluorescent dye (Lucifer Yellow CH, dipotassium salt), followed by 19 extrusions through a 100 nm polycarbonate filter. Elution through a Sephadex G-50 column removed all dye not bound in or on the small vesicles. The fluorescent vesicles were then added to a cancer cell culture in medium and allowed to sit for 40 min. At the end of this period, medium and unbound vesicles were washed away with DPBS, and epifluorescence microscope pictures were taken of the cancer cells (Fig. 3). The cancer cells are seen to display a fluorescence consistent with surface binding of the fluorescent vesicles. As a control experiment, the chemical adhesive 14 was omitted from the vesicles, and the cancer cells failed to display fluorescence. This again suggests the efficacy of the chemical linkage between vesicle and cell. Definite experiments including flow cytometry are being planned. Yet even at this stage of our work the steroidal phosphonate appears to be a promising tool in selective targeting of breast cancer cells.
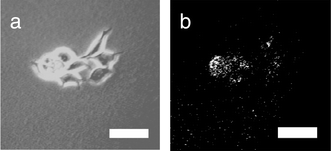 | ||
| Fig. 3 Phase contrast image of MCF-7 cells that have been incubated with submicroscopic vesicles. The vesicles contain adhesive 14 within their membranes and Lucifer Yellow CH in their aqueous interior. (b) Fluorescence image of the same cluster showing fluorescence-labeled cells. Bar = 25 μm. | ||
This work was supported by grant GM21457 from the National Institutes of Health to F. M. Menger.
Notes and references
- K. Von Figura and A. Hasilik, Annu. Rev. Biochem., 1986, 55, 167 CrossRef CAS; S. Kornfeld and I. Mellman, Annu. Rev. Cell Biol., 1989, 5, 483 Search PubMed.
- S. Meresse, U. Bauer, T. Ludwig, F. Mauxion, A. Schmidt and B. Hoflack, Med. Sci., 1993, 9, 148 Search PubMed; Y. Zhao, C. Escot, T. Maudelonde, C. Puech, P. Rouanet and H. Rochefort, Cancer Res., 1993, 53, 2901 Search PubMed.
- B. Hamdaoui, G. Dewynter, F. Capony, J.-L. Montero, C. Toiron, M. Hnach and H. Rochefort, Bull. Soc. Chim. Fr., 1994, 131, 854 Search PubMed; N. Yagi, Y. Ogawa, M. Kodaka, T. Okada, T. Tomohiro, T. Konakahara and H. Okuno, Chem. Commun., l999, 1687 Search PubMed.
- Y. R. Vandenburg, Z.-Y. Zhang, D. J. Fishkind and B. D. Smith, Chem. Commun., 2000, 149 RSC.
- C. Vidil, A. Morere, M. Garcia, V. Barragan, B. Hamdaoui, H. Rochefort and J.-L. Montero, Eur. J. Org. Chem., 1999, 447 CrossRef CAS.
- G. Gregoriadis, N. Engl. J. Med., 1976, 295, 704 CAS; D. D. Lasic, Liposomes: From Physics to Application, Elsevier, Amsterdam, 1993, ch. 14. Search PubMed.
- F. M. Menger and D. E. Johnston, J. Am. Chem. Soc., 1991, 113, 5467 CrossRef CAS; F. M. Menger and K. D. Gabrielson, Angew. Chem., Int. Ed. Engl., 1995, 34, 2091 CrossRef CAS.
- C. C. Kan, J. Yan and R. Bittman, Biochemistry, 1992, 31, 1866 CrossRef CAS.
- V. Barragan, to be published..
| This journal is © The Royal Society of Chemistry 2001 |
