Metal extractions using water in carbon dioxide microemulsions
M. Z.
Yates
a,
D. L.
Apodaca
a,
M. L.
Campbell
a,
E. R.
Birnbaum
b and
T. M.
McCleskey
*a
aActinide, Catalysis, and Separations Chemistry, MS J514, Los Alamos National Laboratory, Los Alamos, NM 87545, USA.. E-mail: tmark@lanl.gov
bAnalytical Chemistry Sciences, MS E518, Los Alamos National Laboratory, Los Alamos, NM 87545, USA
First published on 11th December 2000
Abstract
Water in supercritical carbon dioxide microemulsions are examined as a new medium for the extraction of metal ions from contaminated surfaces, and are shown to extract >99% of the copper from a spiked filter paper with as little as a two-fold excess of surfactant.
Supercritical carbon dioxide (scCO2) has many advantages as a solvent for extractions in environmental remediation including enhanced diffusivity (mass transfer), chemical stability, and pressure-dependent solvation properties that facilitate simple separations. The absence of a liquid–vapor interface above the critical temperature (31 °C) results in zero surface tension, and is particularly advantageous for extraction from solid surfaces since scCO2 may easily penetrate the pores of solid matrices. The challenge for extracting metal ions comes from the fact that supercritical carbon dioxide has no permanent dipole moment and a low dieletric constant (ca. 1.5). Hence, hydrophiles and metal salts typically have near zero solubility. Ligands such as β-diketones, dithiocarbamates, and organophosphorus reagents have been used to solubilize metals into scCO2.1 The solubility of the ligands is quite good in some cases, but solubility of the metal–ligand complexes remains low, limiting practical applications. Solubility of the metal complexes can be enhanced with highly fluorinated ligands,2 but complexes that have moderate CO2 solubility often require a large excess of ligand for efficient extraction.1e,g,i,2e Yazdi and Beckman have shown that attaching ‘CO2-philic’ tails, consisting of either highly fluorinated or polysiloxane groups, to ligands increases the solubility of the metal complexes and results in moderately efficient metal extractions (up to 87%) in liquid CO2.3 The approach of designing specific fluorinated ligands has the drawback that efficient extraction depends on the metal being present as a simple metal salt in a specific oxidation state.
Our approach differs significantly from strategies to date in that we solubilize the metal ion with water in CO2 microemulsions. Johnston et al.4 and Eastoe et al.5 first demonstrated that a perfluoropolyether ammonium carboxylate surfactant was effective in forming water microemulsion droplets (<10 nm in diameter) in supercritical carbon dioxide. We report here on using these water-based microemulsions as a new medium for metal extraction from contaminated surfaces. The nanodroplets of water suspended in CO2 take advantage of both the high solubility of metal ions in water and the high difussivity of CO2 to penetrate pores that are inaccessible to bulk water. The pressure-dependent solvent strength of CO2 can be used to control formation and disruption of the nanodroplets. This extraction scheme is particularly attractive for remediation of heterogeneous waste in which small amounts of metal contaminants are dispersed throughout a large volume of solid waste. Typically, such extractions require an amount of water or solvent proportional to the volume of solid material. With microemulsions, CO2 is effectively used as a diluent and the amount of water need only be proportional to the amount of metal to be extracted, making it possible to decontaminate grams of waste with μL of water.
Water in carbon dioxide microemulsions were formed in a stainless steel variable volume view cell.6 The standard amounts of material added for microemulsion formation were 0.2 g PFPE-NH4, 10 g CO2 and 60 μL water. Selected experiments were done with 0.122 g PFPE-NH4, 10 g CO2 and 42 μL water (data in Table 1). All extractions were conducted at 207 bar and 45 °C. Filter paper was spiked by two different methods. A 42.5 mm diameter filter paper was submerged in aqueous 1 M copper nitrate for at least 48 h (method 1). After drying, the filter paper was cut into quarter sections and extractions were conducted on one section of the paper. For method 2, 5–6 mg of Cu(NO3)2 was dissolved in 30 μL of water and the entire solution was added dropwise to a piece of filter paper. Each filter paper was dried for at least 24 h and placed in a small extraction cell (ISCO, 3 mL cell). The microemulsion was recirculated through the extraction cell at a flow rate of 10 mL min−1. The kinetics of extraction from the filter paper were followed with UV–VIS spectroscopy (Hewlett–Packard model 8453). At the completion of the extraction, at least 50 mL of neat CO2 was passed through the extraction cell (207 bar, 25 °C) to remove residual fluorinated surfactant from the filter paper.The filters were digested and copper concentrations were determined by ICP-AES.
| Number of extractions | Weight of filter paper/g | Concentration, initial/ppm | % Extracted |
|---|---|---|---|
| a Extraction from Whatman No. 3 filter paper; % extracted based on measured value from an unextracted piece of spiked filter paper; method 1 spiking procedure (saturated solution). b Using 50 μL water. c Extraction from Whatman No. 5 filter paper; % extracted based on a calculated value for 5 and 6 mg Cu(NO3)2; method 2 spiking procedure. | |||
| 1a | 0.018 | 81400 | 99.65 |
| 1a,b | 0.019 | 81400 | 99.53 |
| 1c | 0.384 | 3430 | 58.5 |
| 2c | 0.386 | 4090 | 98.0 |
| 2c | 0.238 | 6630 | 82.8 |
| 2c | 0.096 | 16400 | 99.7 |
The extinction coefficient for the 733 nm band of Cu(NO3)2 in the water/CO2 microemulsion was determined to be 59.3 M−1 cm−1.7 The low energy absorption band is blue shifted compared to copper nitrate in nitric acid (810 nm) at ambient conditions (Fig. 1). We attribute this shift to two factors: copper binding to the surfactant head groups and a decrease in the polarity of water in the micelle core. The maximum solubility of copper in the microemulsion was found to be 3 M.
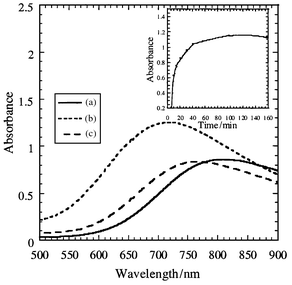 | ||
| Fig. 1 The absorption spectra of copper(II) from: (a) copper nitrate dissolved in nitric acid at ambient conditions, (b) copper nitrate dissolved in the water/CO2 microemulsion, and (c) aqueous copper acetate at ambient conditions. The inset shows the intensity at 733 nm vs. time for extraction of copper. | ||
Extractions of copper from spiked filter paper were conducted to examine the effectiveness of the microemulsion in removing metal contaminates from solid surfaces (Table 1). More than 99% of the copper is extracted in one step from smaller pieces of filter paper. Extraction efficiency decreases when a larger piece of filter paper is used, but following with a second extraction improves the efficiency to 82–99%. The kinetics of extraction from a spiked filter paper (method 1) were followed by UV–VIS spectroscopy (Fig. 1). The absorption from copper reached a maximum after 60 min of exposure to the microemulsion, with no further increase after an additional 90 min. The kinetics of extraction with microemulsions are much faster than those observed recently with CO2-soluble polymers that required many hours to days for extraction.8 In addition to improved kinetics, the microemulsions have improved efficiency compared to previous studies with CO2-soluble chelating agents. In general, past extractions have required a tremendous excess of ligand to achieve reasonable metal extractions (>1000 fold).1 By comparison, at the maximum solubility of copper, the molar ratio of surfactant to copper in the microemulsion is approximately 3.
Europium fluorescence lifetimes and emission spectra were examined to probe ion solvation in the water in CO2 microemulsions. The relative heights of the europium emission bands at 593 and 615 nm provide information on the first solvation sphere around the europium ion.9,10 In the microemulsion, the 615 nm band gains in intensity, indicating that the center of inversion around europium is removed by displacement of water in the first hydration sphere by another ligand.11 Two possible ligands are available in the microemulsion core: the carboxylate head group of the surfactant or carbonic acid, formed by CO2 dissolved in the aqueous phase. As a control, europium chloride was dissolved in D2O and the headspace over the solution was pressurized to 3000 psi with carbon dioxide to introduce bicarbonate with no surfactant present. The europium emission spectra appeared the same as in water at ambient conditions, suggesting that the carboxylate head group of the surfactant is solely responsible for the changes in the emission spectra of europium ion dissolved in the microemulsion.
Futher evidence for surfactant binding comes from lifetime experiments using H2O- and D2O-based microemulsions. The isotope effect provides a way to determine the number of water molecules surrounding the europium ion using the equation: n = C*(1/τH − 1/τD), where n is the number of water molecules, C is a constant (1.05 for Eu), τH is the fluorescenece lifetime in H2O, and τD is the fluorescence lifetime in D2O (lifetimes in milliseconds).9 The lifetime of europium(III) ion in the water in supercritical CO2 microemulsion was found to be 120 μs and increased to 274 μs when the microemulsion was formed with D2O. Using the above equation with the measured lifetimes gives ca. 5 water molecules in the first hydration sphere of the europium ions. As the fully hydrated ion has 8 to 9 water molecules,12 this result suggests that the surfactant displaces 3 or 4 water molecules from around the europium ion.
Radioactive and heavy metal contaminants are often present as water-insoluble metal oxides in heterogeneous waste. To demonstrate the versatility of the microemulsion in metal extraction, the microemulsion was formed with a 20 wt% nitric acid solution instead of pure water. The increase in acidity was confirmed by dissolving methyl orange indicator in the microemulsion. The indicator appeared bright red when the microemulsion was formed with 20% nitric acid as opposed to orange when distilled water was used. The ability of the acidic microemulsions to extract metal oxides was examined with europium oxide. Based upon visual observation, 10 mg of europium oxide completely dissolved into a microemulsion formed with 20 wt% nitric acid after stirring for 1 h.
Water in supercritical carbon dioxide microemulsions are effective for extraction of metals from solids. At maximum solubility, a 3 M solution of copper can be obtained within the microemulsion core. The nanodroplets offer the advantages of rapid and efficient extractions with a versatile environment. Making the microemulsion with 20 wt% nitric acid rather than pure water allowed europium oxide to be solubilized. Agents that oxidize low valent metal oxides or water-soluble chelating agents could also be introduced into the microemulsions to expand the types of metals that may be extracted and to provide selectivity in mixed metal extractions. Metal is readily recovered by simply dropping the CO2 pressure below the cloud point, causing the water to coalesce into a single droplet with all of the metal and some surfactant. Initial experimental work has indicated that surfactant recycle should be possible by adding excess water and reducing pressure to destabilize the microemulsion while maintaining surfactant solubility. An additional 270 μL of water was added to a copper-saturated microemulsion at 207 bar, and then pressure was reduced to 101 bar. At 101 bar, phase separation occurred and the aqueous phase settled to the bottom of the cell, while a significant fraction of the surfactant remained in the upper phase. No copper was observable in the upper phase by UV–VIS. The ability of the microemulsion to concentrate the metal into a small volume of water makes it particularly attractive for extractions from contaminated solids that often have small amounts of metal dispersed over a large volume of solid waste.
Notes and references
- (a) J. Darr and M. Poliakoff, Chem. Rev., 1999, 99, 495 CrossRef CAS; (b) Y. Meguro, S. Iso and Z. Yoshida, Anal. Chem., 1998, 70, 1262 CrossRef CAS; (c) Y. Meguro, S. Iso, T. Sasaki and Z. Yoshida, Anal. Chem., 1998, 70, 774 CrossRef CAS; (d) N. G. Smart, T. Carleson, T. Kast, A. A. Clifford, M. D. Burford and C. M. Wai, Talanta, 1997, 137 CrossRef CAS; (e) Y. Lin, N. G. Smart and C. M. Wai, Environ. Sci. Technol., 1995, 29, 2706 CAS; (f) A. F. Lagalante, B. N. Hansen, T. J. Bruno and R. E. Sievers, Inorg. Chem., 1995, 34, 5781 CrossRef CAS; (g) S. Iso, Y. Meguro and Z. Yoshida, Chem. Lett., 1995, 365 CAS; (h) K. E. Laintz and E. Tachikawa, Anal. Chem., 1994, 66, 2190 CrossRef CAS; (i) J. Wang and W. D. Marshall, Anal. Chem., 1994, 66, 1658 CrossRef CAS.
- (a) C. M. Wai and S. J. Wang, Chromatogr. A., 1997, 785, 369 CrossRef CAS; (b) M. Z. Özel, M. D. Burford, A. A. Clifford, K. D. Bartle, A. Shadrin, N. G. Smart and N. D. Tinker, Anal. Chim. Acta, 1997, 346, 73 CrossRef; (c) J. M. Murphy and C. Erkey, Ind. Eng. Chem. Res., 1997, 36, 5371 CrossRef CAS; (d) J. D. Glennon, S. Hutchinson, A. Walker, S. J. Harris and C. C. McSweeney, J. Chromatogr. A, 1997, 770, 85 CrossRef CAS; (e) K. E. Laintz, C. M. Wai, C. R. Yonker and R. D. Smith, J. Supercrit. Fluids, 1991, 4, 194 CrossRef CAS.
- (a) A. V. Yazdi and E. J. Beckman, J. Mater. Res., 1995, 10, 530 CAS; (b) A. V. Yazdi and E. J. Beckman, Ind. Eng. Chem. Res., 1996, 35, 3644 CrossRef CAS; (c) A. V. Yazdi and E. J. Beckman, Ind. Eng. Chem. Res., 1997, 36, 2368 CrossRef CAS.
- K. P. Johnston, K. L. Harrison, M. J. Clarke, S. M. Howdle, M. P. Heitz, F. V. Bright, C. Carlier and T. W. Randolph, Science, 1996, 271, 624 CrossRef CAS.
- J. Eastoe, B. M. H. Cazelles, D. C. Steytler, J. D. Holmes, A. R. Pitt, T. J. Wear and R. K. Heenan, Langmuir, 1997, 13, 6980 CrossRef CAS.
- M. Z. Yates, G. Li, J. J. Shim, S. Maniar, K. P. Johnston, K. T. Lim and S. Webber, Macromolecules, 1999, 32, 1018 CrossRef CAS.
- The extinction coefficient was determined by dissolving Cu(NO3)2 in the microemulsion and assuming a homogeneous environment..
- K. Powell, T. M. McCleskey, W. Tumas and J. DeSimmone, Macromolecules, submitted..
- W. D. Horrocks and D. R. Sudnick, J. Am. Chem. Soc., 1979, 101, 334 CrossRef CAS.
- J.-C. G. Bunzli and J. Yersin, Inorg. Chem., 1979, 18, 605 CrossRef.
- J.-C. G. Bunzli, in Lanthanide Probes in Life, Chemical and Earth Sciences, ed. J.-C. G. Bunzli and G. R. Choppin, Elsevier, Amsterdam, 1989, ch. 7, p. 219. Search PubMed.
- W. Grygiel and M. Starzak, J. Lumin., 1997, 71, 21 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
