Sono-emulsion electrosynthesis: electrode-insensitive Kolbe reactions
Jay D. Wadhawana, Frank Marken†a, Richard G. Compton*a, Steven D. Bull‡b and Stephen G. Daviesb
aPhysical and Theoretical Chemistry Laboratory, Oxford University, South Parks Road, Oxford, UK OX1 3QZ. E-mail: compton@ermine.ox.ac.uk
bDyson Perrins Laboratory, Oxford University, South Parks Road, Oxford, UK OX1 3QY
First published on 14th December 2000
Abstract
The electro-oxidation of water-immiscible liquid aliphatic acids (RCO2H) leading to decarboxylation to afford a hydrocarbon (R–R) may be achieved using an emulsion formed via insonation so that the organic phase continuously extracts the products; in complete contrast to conventional monophasic electrolyses, the type and yield of products obtained from this biphasic Kolbe electrolysis are independent of the electrode material used.
The introduction of power ultrasound into homogeneous solutions has a considerable effect upon mass transport processes due to macroscopic streaming and microscopic interfacial cavitational events;1 the sonication of biphasic media can result in the formation of an in situ emulsion,2–4 primarily due to cavitational events that occur preferentially at the phase boundary.3 These mechanical forces act to divide droplets again and again forming microdroplets and effectively ‘homogenising’ the heterogeneous system.
Electrosynthesis in emulsion media is long established.5,6 Sono-emulsion systems have considerable potential for application in electrosynthesis because (i) there is no need for emulsion stabilising reagents, (ii) the separation of products is facile, (iii) aqueous solutions may be co-emulsified with a sparingly soluble liquid depolariser, resulting in a medium of high conductivity, (iv) high rates of mass transport can be achieved, and (v) the electrode surface is continually activated.7
We report the use of sono-emulsion media to study the electro-oxidation of hexanoic acid (Kolbe reaction). Previous sono-electrosynthetic studies8–12 have focussed upon the effects of ultrasound on Kolbe electrolysis, exclusively under monophasic conditions, and with seemingly contradictory data. In this work it is shown that under biphasic conditions, although Kolbe electrolysis is conducted at a potential far beyond that required for solvent decomposition, an efficient conversion of hexanoic acid into decane is achieved at both platinum and boron-doped CVD (chemical vapour deposited) diamond film anodes. Surprisingly, the anode material does not affect the type or ratio of products formed.
An electrochemical cell equipped with an ultrasonic probe§ was used as reported earlier.13 With an ultrasound intensity of 190 W cm−2, this configuration leads to forceful mixing and emulsification of two-phase systems such as water | decane. The biphasic emulsion systems of hexanoic acid (ca. 5 mL) in aqueous 1.0 M NaOH (ca. 15 ml) had a conductivity of ca. 20 mS cm−1 and pH of 6.1. These values were kept more or less constant throughout the electrolysis by the reduction of water at the platinum counter electrode, and the gradual dissolution of the organic acid into the aqueous solution phase.
Power ultrasound was used to emulsify ca. 15 mmol of hexanoic acid in an aqueous 1.0 M NaOH biphasic system. After the passage of 1 Faraday (per mole of hexanoic acid) of charge (ca. 1500 C, sufficient to quantitatively convert hexanoic acid assuming a one-electron oxidation process) the reaction was stopped, excess hydroxide neutralised with acid, and the organic products extracted with ethyl acetate and analysed by GC–MS and NMR spectroscopy. Data summarised in Table 1 describe the conditions used and the yields of the main product, decane—the Kolbe dimer, generated in this electrolysis process. At platinum electrodes, it can be seen that a threshold current density (or applied potential) exists below which the Kolbe process cannot compete against background processes such as oxygen evolution. As in the monophasic case,14 increasing the temperature at which the reaction takes place causes a decrease in current efficiency; decreasing the aqueous electrolyte concentration also has this effect. The effect of the current density can be rationalised as follows. Initially, increasing the current density causes the current efficiency to improve, and good yields of decane are observed at current densities in the range 0.2–0.3 A cm−2; at values of 0.35 A cm−2 and higher, the current efficiency decreases. This can be rationalised in terms of the flux of hexanoate towards the electrode surface. The ultrasound-determined mass transport limiting current (Ilim) in homogeneous solution is given by1 eqn. (1):
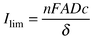 | (1) |
| Mass of hexanoic acid/g | Concentration of NaOH/M | Current density/ A cm−2 | Tenperature/K | Yieldb (%) of decane |
|---|---|---|---|---|
| a Reaction conditions: oxidation of hexanoic acid at a 1.1 cm2 platinum anode (or at a 0.25 cm2 free-standing polycrystalline boron-doped CVD diamond electrode) in an aqueous NaOH/hexanoate emulsion system in the presence of power ultrasound (7 mm electrode-to-horn distance, 190 W cm−2). Charge equivalent to 1 Faraday per mol of hexanoic acid was passed before analysis (ca. 1500 C).b Yields based upon quantitative analysis of 1H NMR signals. | ||||
| 1.1 cm2 platinum disc anode | ||||
| 1.72 | 1.0 | 0.08 | 293 | 0 |
| 1.72 | 1.0 | 0.13 | 293 | 24 ± 3 |
| 1.83 | 1.0 | 0.18 | 293 | 45 ± 5 |
| 1.82 | 1.0 | 0.35 | 293 | 40 ± 5 |
| 1.67 | 0.1 | 0.18 | 293 | 17 ± 2 |
| 1.87 | 1.0 | 0.18 | 313 | 3 ± 1 |
| 0.25 cm2 free-standing polycrystalline boron-doped CVD diamond anode | ||||
| 1.80 | 1.0 | 0.35 | 293 | 40 ± 5 |
| 1.80 | 1.0 | 0.70 | 293 | 14 ± 5 |
The Kolbe dimer is not produced exclusively: GC–MS and NMR analysis of the electrolytic products permits the identification of the ester, amyl caproate, formed in <5% yield. The absence of any pentenes and pentanols is novel and surprising. This is indicative of a trapping of the initial reaction intermediates at the electrode surface; the pentyl carbocation is unlikely to be formed as a free intermediate during electrolysis.
It is widely known that ultrasound damages the surface of platinum electrodes.1 Recently, boron-doped CVD diamond electrodes have been employed in the presence of ultrasound with negligible damage to the electrode surface.16 Furthermore, diamond surfaces are chemically inert under hostile conditions and after long-term electrolysis at very positive potentials.17 The level of boron doping is high, typically ca. 1020 cm3, corresponding to a B∶C atom ratio of 1∶1000, allowing a resistivity of 0.3 mΩ m to be achieved.18
Galvanostatic electrosynthesis experiments employing a boron-doped CVD diamond anode were conducted at different current densities, and the products analysed as before. The products observed in conventional Kolbe electrolyses suggest that the electrode material exerts a strong control, with products predominantly derived from carbocation intermediates detected at carbon anodes.14 Surprisingly, electrosynthesis at a boron-doped CVD diamond anode under sono-emulsion conditions again gives rise to the detection of predominately the Kolbe dimer (see Table 1). Current efficiencies and yields are only slightly lower than those observed at platinum electrodes. Interestingly, the ester, amyl caproate is again the sole by-product, suggesting that a mechanism similar to that at platinum electrodes is operative.
In summary, Kolbe electrolysis has been accomplished under the novel conditions of an emulsion generated in situ by power ultrasound. This method of electrosynthesis renders good yields of product and is highly charge efficient. For the first time in 150 years,19 the electrode material used and conditions employed are observed to have only little effect upon the type of products formed. Although the mechanism of the reaction is unclear, it may involve encapsulation of intermediates within the organic component of the emulsion. This type of electrosynthetic methodology shows promise for wider application.
Acknowledgements
We thank the Royal Society for financial support through a University Research Fellowship (F. M.), and DeBeers Industrial Diamond Division, UK and the EPSRC (GR/N 12051) for supporting this work.Notes and references
- For a review see F. Marken, J. C. Eklund and R. G. Compton, Electroanalysis, 1997, 7, 509.
- F. Marken, R. G. Compton, S. D. Bull and S. G. Davies, Chem. Commun., 1997, 955 RSC.
- F. Marken and R. G. Compton, Electrochim. Acta, 1998, 43, 2157 CrossRef CAS.
- O. Behrend, K. Ax and H. Schubert, Ultrason. Sonochem., 2000, 7, 77 CrossRef CAS.
- H. Fees and H. Wednt, in Techniques of Electroorganic Chemistry Part III, ed. N. L. Weinberg, Wiley, New York, 1981, p. 81. Search PubMed.
- J. F. Rusling and D. L. Zhou, J. Electroanal. Chem., 1997, 439, 89 CrossRef CAS.
- R. P. Akkermans, S. L. Roberts and R. G. Compton, Chem. Commun., 1999, 1115 RSC.
- H. Fujiwara, M. Atobe, H. Kanetsuna and T. Nonaka, J. Chin. Chem. Soc., 1998, 45, 175 Search PubMed.
- M. Tashiro, H. Tsuzuki, H. Goto and S. Makata, Chem. Exp., 1991, 4, 41 Search PubMed.
- D. J. Walton, S. S. Phull, U. Geissler, A. Chyla, A. Durham, S. Ryley, T. J. Mason and J. P. Lorimer, Electrochem. Commun., 2000, 2, 431 CrossRef CAS.
- A. Chyla, J. P. Lorimer, T. J. Mason, G. Smith and D. J. Walton, J. Chem. Soc., Chem. Commun., 1989, 603 RSC.
- D. J. Walton, A. Chyla, J. P. Lorimer and T. J. Mason, Synth. Commun., 1990, 1843 CAS.
- A. N. Blythe, R. P. Akkermans and R. G. Compton, Electroanalysis, 2000, 12, 16 CrossRef CAS.
- C. J. Brockman, Electroorganic Chemistry, Wiley, New York, 1926. Search PubMed.
- C. R. Wilke and P. Chang, AIChE J., 1955, 1, 264 Search PubMed.
- C. H. Goeting, J. S. Foord, F. Marken and R. G. Compton, Diamond Rel. Mater., 1999, 8, 824 Search PubMed.
- P. A. Michaud, E. Mahe, W. Haenni, A. Perret and C. Comninellius, Electrochem. Solid State Lett., 2000, 3, 77 CrossRef CAS.
- R. G. Compton, F. Marken, C. H. Goeting, R. A. J. McKeown, J. S. Foord, G. Scarsbrook, R. S. Sussmann and A. J. Whitehead, Chem. Commun., 1998, 1961 RSC.
- H. Kolbe, Ann. Chim., 1849, 69, 257 Search PubMed.
Footnotes |
| † Present address: Department of Chemistry, Loughborough University, Loughborough, UK LE11 3TU. |
| ‡ Present address: School of Chemistry, University of Bath, Claverton Down, Bath, UK BA2 7AY. |
| § The ultrasound generator employed was a VCX400 model immersion horn (Sonics and Materials, USA) equipped with a 3 mm diameter stepped titanium-alloy tip (electrically insulated) emitting 20 kHz sound with power level set to 190 W cm−2 (calorimetrically determined). The horn-to-electrode distance was maintained at 7 mm. Galvanostatic electrosynthesis was undertaken in the thermostatted cell (volume ca. 20 cm3) using a PAR 173 (EG&G) galvanostat fitted with a PAR 178 (EG&G) digital coulometer. The cathode employed was a coil of platinum wire; the anode was either a 12 mm diameter platinum disc (Aldrich) or a 5 × 5 mm free-standing polycrystalline boron-doped CVD diamond plate (DeBeers Industrial Diamond Division, UK). Chemical reagents and NaOH (Aldrich) were of the highest commercially available purity. |
| This journal is © The Royal Society of Chemistry 2001 |
