Responsive nanocapsules
Marc
Sauer
and
Wolfgang
Meier
*
Department of Physical Chemistry, University of Basel, Klingelbergstrasse 80, CH-4056, Basel, Switzerland.. E-mail: wolfgang.meier@unibas.ch
First published on 14th December 2000
Abstract
Using vesicular polymerization, water-soluble polyelectrolyte nanocapsules have been prepared, that are able to undergo a reversible swelling transition upon changing the pH and/or salt concentration.
In recent years considerable progress has been made in developing synthetic methods that allow preparation of materials with precise control over size and morphology at the nanometer level. One typical example is the formulation of hollow nanoparticles. For such nanocontainers a widespread range of applications such as confined reaction vessels, drug carriers or protective shells for cells or enzymes have been proposed. Similar and very effective nanometer sized containers, viz., micelles and vesicular structures, are already used by nature in biological systems. However, due to the non-covalent interactions responsible for their formation, these objects have only a limited stability and may undergo structural changes. Many applications (e.g. in drug delivery) require more stable particles. Mechanically stable polymer nanocapsules can be prepared using a variety of different techniques (see for example refs. 1–9). In view of their high stability and low permeability it is, however, generally rather difficult to load polymeric capsules after formulation or to release substances from their interior in a controlled way at the desired target. Consequently prior to any application of such polymer shells ways have to be found to control their permeability.10 One possibility to realize this is nicely demonstrated by a naturally occurring polymeric nanocontainer: the protein shells of the cowpea chlorotic mottle virus (CCMV) show a reversible, pH-induced structural transition.11 Increasing the pH from ca. 5 to 7 leads to a swelling of the protein shells by ca. 10%. During this swelling gated pores are opened in their shells which allow free molecular exchange between the interior of the virion cages and the bulk medium. Recently this gating transition of the virion particles has been used for host–guest encapsulation.12 Practical applications of virion cages on a technical scale are not feasible due to the difficulties in handling and producing larger quantities. Here our aim is to find a fairly simple synthetic mimetic of these virion cages able to undergo a similar structural transition.
The conformation of polyelectrolytes is very sensitive towards changes in ionic strength, pH and other external factors. Hence water-soluble polymer hollow spheres formed by a covalently crosslinked polyelectrolyte shell could be a suitable model system. The carboxylate groups of nanocapsules based, for example, on poly(acrylic acid) dissociate increasingly with increasing pH. As a result of the associated electrostatic repulsion between the identically charged carboxylate anions along the polymer backbone the particles should swell considerably. Similar to the virion cages, the permeability of the swollen poly(acrylate) shells should increase and enhance molecular exchange between the interior of the particles and the bulk medium. Here we describe a synthetic route to nanometer-sized poly(acrylic acid) hollow spheres. To demonstrate their potential for host–guest encapsulation and controlled release their swelling behavior in aqueous media of different pH and ionic strength, has been investigated.
Recently we succeeded in preparing hydrophobic polymer hollow spheres with diameters ranging from several nanometers up to hundreds of micrometers by polymerization of hydrophobic monomers in the lipid bilayer of vesicles or liposomes.5 While the size and shape of the resulting polymer particles are directly determined by the templating vesicles, the polymer scaffold can be modified fairly easily, using conventional chemical reactions.
The preparation of hydrophobic polymer hollow spheres has already been described elsewhere.5 Therefore we just recall briefly the individual steps. We prepared small unilamellar vesicles from the synthetic surfactant dimethyldioctadecylammonium chloride (DODAC) by ultrasonification of aqueous lipid dispersions (2.1 g DODAC in 97.9 g bidistilled water). This yields unilamellar vesicles with an average diameter of 100 nm, however, with a rather high polydispersity. The lipid bilayers of the vesicles were swollen by incubating them in the presence of hydrophobic monomers (0.52 g, i.e., 25 wt% with respect to the lipid) for 2 h at 60 °C. A crosslinking polymerization of the monomers was initiated by UV-irradiation (2 h) at room temperature. Afterwards the resulting polymer particles were isolated from the vesicles by repeated precipitation in a large excess of methanol–water (3∶1) until 1H NMR spectroscopy indicated complete removal of the surfactant (yield: 81%). As hydrophobic monomers we used mixtures of tert-butyl acrylate (t-BUA) and ethylene glycol dimethacrylate (EGDMA) as the crosslinking agent. For selective saponification of the tert-butyl ester groups13 the crosslinked poly(tert-butylacrylate) hollow spheres were dissolved in dioxane and stirred for 8 h at 80 °C in the presence of a catalytic amount of hydrochloric acid. The resulting poly(acrylic acid) particles were precipitated in diethyl ether and finally dried by lyophilization from dioxane (yield 89%). 1H NMR spectroscopy indicates full conversion of the poly(tert-butyl acrylate) under these conditions.
The resulting poly(acrylic acid) particles are dispersable in aqueous media. Fig. 1 shows a characteristic cryogenic electron micrograph (cryo-TEM) of a representative sample in an aqueous phosphate buffer at pH = 7. The micrograph shows clearly that the polymer particles are hollow. Although we cannot completely exclude their presence due to the limited resolution of the method, no solid latex particles or fragments of the shells can be detected. The size of the particles is in good agreement with dynamic light scattering experiments (see below) and they are polydisperse as are the parent DODAC vesicles. This reflects that the newly formed poly(acrylic acid) hollow spheres have survived intact the isolation and saponification procedure.
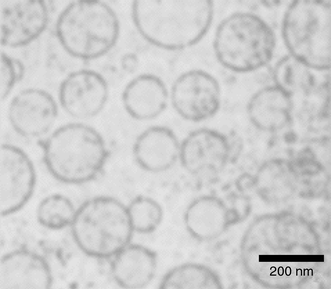 | ||
| Fig. 1 Cryo-transmission electron micrograph of poly(acrylic acid) hollow spheres in an aqueous phosphate buffer at pH = 7. | ||
The sensitivity of the particles towards changes of the pH and/or ionic strength of the buffer is the basic requirement for their use as controlled release devices. Therefore we investigated in a first set of experiments the behavior of the poly(acrylic acid) hollow spheres in phosphate buffers of varying pH. It has to be emphasized that the ionic strength of the buffers used for these experiments was carefully kept constant at 0.1 M by addition of sodium chloride. Although electrostatic interactions are shielded considerably this salt concentration is close to physiological conditions (ca. 150 mM), the relevant range for most pharmaceutical applications. Additionally the systems are quite insensitive towards small ionic impurities at this concentration.
The pH-induced dimensional changes of the particles were investigated by dynamic light scattering and Fig. 2 shows the results. To take into account small variations in the average radius of different batches of the precursor vesicles we plotted a reduced hydrodynamic radius Rh/Rh,0 (Rh = hydrodynamic radius of poly(acrylic acid) hollow spheres; Rh,0 = hydrodynamic radius of the polymer containing vesicular precursors) as a function of pH. As can directly be seen the dimensions of the particles increase considerably with increasing pH. In the range pH 4–8 their radius increases by a factor of about 4 (i.e., their encapsulated volume increases by a factor of 64!). It should be emphasized that this swelling is completely reversible. The different curves in Fig. 2 refer to nanocapsules of different crosslinking density which was controlled by the molar fraction of the crosslinker EGDMA in the particles. With increasing crosslinking density, the maximum swelling decreases and the swelling transition is shifted towards higher pH, which is a result of the increasing fraction of the non-ionic EGDMA comonomers. The same effect has also been observed for other polyelectrolyte gels.14 It is important to note that the poly(acrylic acid) hollow spheres precipitate at low pH. The first points of the different curves in Fig. 2 represent the lowest pH values for which stable particle dispersions were obtained. Such a pH-induced precipitation could be highly interesting for applications since it represents a convenient way to separate the particles after their loading from the solution.
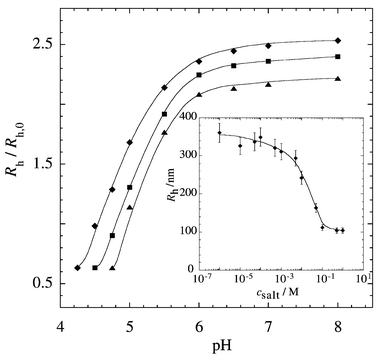 | ||
| Fig. 2 Reduced hydrodynamic radius Rh/Rh,0 as a function of pH. The different curves correspond to particles with a different molar fraction of crosslinking comonomers: (◆) 3 mol% EGDMA (Rh,0 = 49 nm), (■): 5 mol% EGDMA (Rh,0 = 55 nm), (▲) 10 mol% EGDMA (Rh,0= 60 nm). Inset: hydrodynamic radius Rh of poly(acrylic acid) hollow spheres (5 mol% EGDMA) as a function of salt concentration csalt of the buffer. | ||
The effect of varying salt (NaCl) concentration is shown in the inset of Fig. 2. Throughout this series of measurements we kept the pH constant at 6 where the poly(acrylic acid) is highly ionized. As can directly be seen with decreasing salt concentration the swelling of the particles increases and tends towards a saturation value, which is due to decreasing electrostatic shielding effects. Similar observations have been made with macroscopic poly(acrylic acid) based gels.15 It is interesting that we can conclude from these data an increase of the radius of the poly(acrylic acid) hollow spheres by up to a factor of 10 (i.e. the encapsulated volume increase by a factor of 1000!) at low salt concentrations.
In conclusion, we have presented a convenient way to prepare new, pH-sensitive nanocapsules based on crosslinked polyelectrolyte shells. These particles undergo a reversible swelling transition upon changing the pH and/or the salt concentration of the system which reversibly changes their dimensions. We expect that this swelling will have considerable influence on the permeability of the shells of these nanocapsules which makes these systems highly interesting for applications in areas such as drug delivery. Initial preliminary results indicate that this ‘gating transition’ can successfully be used for controlled encapsulation and release of biopolymers such as enzymes.
Acknowledgements
Financial support from the Swiss National Science foundation is gratefully acknowledged.Notes and references
- J. Murtagh and J. K. Thomas, Faraday Discuss. Chem. Soc., 1986, 81, 127 RSC.
- J. Kurja, R. J. M. Noelte, I. A. Maxwell and A. L. German, Polymer, 1993, 34, 2045 CrossRef CAS.
- J. D. Morgan, C. A. Johnson and E. W. Kaler, Langmuir, 1997, 13, 6447 CrossRef CAS.
- J. Hotz and W. Meier, Langmuir, 1998, 14, 1031 CrossRef CAS.
- E. Donath, B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem., Int. Ed., 1998, 37, 2201 CrossRef.
- H. Huang, E. E. Remsen, T. Kowalewski and K. L. Wooley, J. Am. Chem. Soc., 1999, 121, 3805 CrossRef.
- S. Stewart and G. J. Liu, Chem. Mater., 1999, 11, 1048 CrossRef CAS.
- O. Emmerich, N. Hugenberg, M. Schmidt, S. S. Sheiko, F. Baumann, B. Deubzer, J. Weis and J. Ebenhoch, Adv. Mater., 1999, 11, 1299 CrossRef CAS.
- C. Nardin, T. Hirt, J. Leukel and W. Meier, Langmuir, 2000, 16, 1035 CrossRef CAS.
- C. Nardin, S. Thoeni, J. Widmer, M. Winterhalter and W. Meier, Chem. Commun., 2000, 1433 RSC.
- J. A. Speir, S. Munshi, G. Wang, T. S. Baker and J. E. Johnson, Structure, 1995, 3, 63 CrossRef CAS.
- T. Douglas and M. Young, Nature, 1998, 393, 152 CrossRef CAS.
- C. Ramireddy, Z. Tuzar, K. Prochazka, S. E. Webber and P. Munk, Macromolecules, 1992, 25, 2541 CrossRef CAS.
- R. A. Siegel, in Responsive Gels: Volume Phase Transitions I & II, in Advances in Polymer Science, ed. K. Dusek, Springer, Berlin, 1992/1993, vol. 109/110, p. 234. Search PubMed.
- J. Ricka and T. Tanaka, Macromolecules, 1984, 17, 2916 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
