One-step self-assembly organometallic molecular cages from 11 components†
Shih-Sheng Sun and Alistair J. Lees*
Department of Chemistry, State University of New York at Binghamton, Binghamton, NY 13902-6016, USA.. E-mail: alees@binghamton.edu
First published on 15th December 2000
Abstract
Two self-assembly organometallic molecular cages, each comprising 11 components and two different bridging ligands, were prepared from one-step reactions; both of these cages exhibit relatively high affinity and shape-selectivity toward planar aromatic compounds.
Self-assembly has been recognized as a most efficient process that organizes individual molecular components into highly ordered supramolecular species.1 Relying on strong metal–ligand interactions, the design and study of well arranged metal-containing macrocycles or three-dimensional cage molecules has emerged as a promising research area in modern supramolecular chemistry.2
To date, most of the one-step self-assembly transition-metal containing supramolecular species reported in the literature have been synthesized from the same metal components and bridging ligands.2,3 The incorporation of different metal components or more than two different types of bridging ligands are much rarer.4 In most cases, such supramolecules were prepared from two or more steps.5
Recently, we have prepared a series of self-assembly molecular squares and triangles that incorporate photoactive chromophores. We have demonstrated that the geometry, length and electronic properties of the bridging ligand play an important role in determining the shape of the final self-assembly products and their resultant photophysical, photochemical and electrochemical properties as well as their binding capabilities in molecular sensing applications.3a,b,6 As an extension of our previous work, we set out to design and synthesize self-assembly cage-type molecules that comprise two different types of ligands, which require more precise bonding directions between the metal components and the bridging ligands in order to avoid the formation of undesired oligomeric species. During the progress of our work, two communications have appeared that describe the two-step preparation of rectangular structures utilizing 2,2′-bipyrimidine (BPM) or bisbenzimidazolate and 4,4′-bipyridine as bridging units.5a,d‡ We report, herein, the synthesis of two new cage molecules, each involving two different bridging ligands and assembled from eleven individual components, including a route that involves only one stage and yet leads to high yield. Their photophysical properties and binding behavior toward electron-rich aromatic compounds are also described.
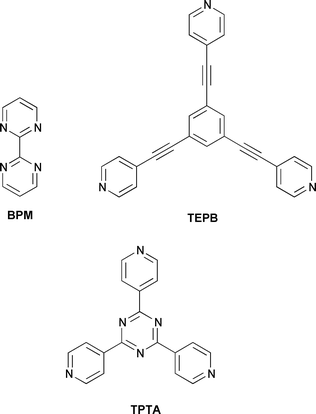
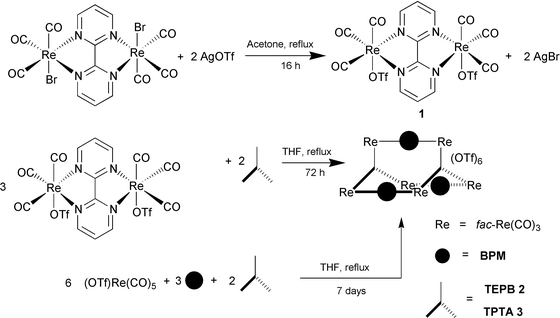
Two synthetic routes to these cage molecules have been investigated (see Schemes 1 and 2). The first involved synthesis of the BPM bridged Re(I) dimer and subsequent replacement of the bromo ligand by triflate (OTf) in acetone to isolate complex 1.5d Subsequent reflux of 1 and the corresponding tridentate ligands, 1,3,5-tris(2′-ethynyl-4″-pyridyl)benzene (TEPB)7 or 2,4,6-tris(4′-pyridyl)-1,3,5-triazine (TPTA)8 in THF for 3 days afforded reddish products with general formula {[fac-Re(CO)3]2(μ-BPM)}3 (μ-L)2(OTf)6 (2, L = TEPB; 3, L = TPTA).†§
 | ||
| Scheme 1 | ||
 | ||
| Scheme 2 | ||
Cages 2 and 3 were characterized by several different analytical techniques including IR, NMR, elemental analysis, and electrospray ionization mass spectrometry (ESI-MS). IR spectra of both cages 2 and 3 indicate typical tricarbonyl patterns with facial arrangements.9 Elemental analyses confirmed the proposed stoichiometry. 1H NMR spectra of both cages 2 and 3 exhibit very clean and simple chemical shifts, ruling out the possible existence of other oligomeric species. The ESI-MS measurements have provided a straightforward identification of the cage structures of 2 and 3.† The excellent agreement between the observed and simulated isotopic distributions for the molecular ion unambiguously confirms the proposed molecular structures for 2 and 3.
After successful isolation of the desired cages 2 and 3, we wondered if it was possible to prepare them in a simpler one-step process. The synthesis was initiated by refluxing (OTf)- Re(CO)5, BPM and L (TEPB or TPTA) in a 6∶3∶2 ratio in THF for one week and, subsequently, cages 2 and 3 were isolated in 47 and 41% yield, respectively. The BPM bridged dimer, [(CO)3ReOTf]2(μ-BPM), would be expected to form first in the solution considering the extra stabilization through the chelation effect of BPM. Indeed, the formation of one precursor, which is more thermodynamically stable than the other possible intermediates, is important for a self-assembly process that comprises more than one bridging ligand. Subsequently, the thermodynamically driven self-assembly process between [(CO)3ReOTf]2(μ-BPM) and the corresponding TEPB and TPTA ligands afforded the final cage products.
The absorption spectra of 2 and 3 in MeCN feature intense bands in the near-UV region and a low-energy band at 470 nm, tailing past 600 nm.§ This low energy band is assigned to the Re(dπ) to BPM (π*) charge-transfer transition (MLCT). Both 2 and 3 are non-emissive in room-temperature MeCN solution. Several BPM-bridged Re(I) tricarbonyl complexes have been reported that exhibit a lack of emission.5d,10 These observations have been attributed to the energy gap law effect,11 resulting in the low-energy 3MLCT excited state undergoing very efficient nonradiative decay. However, it is also possible that an emission band is present but is too red-shifed to detect on our instrumentation.
According to MM2 molecular modeling results, the interplanar distances between the two tridentate ligands in 2 and 3 are 3.5 and 4.2 Å, respectively.12 These interplanar distances are comparable to the recently reported rectangular structures5a,d and the molecules are considered to have very effective π–π stacking that contributes to the highly thermodynamically stable structures.4a,c The nearly co-planar arrangement between the two tridentate ligands and the overall six positive charges in 2 and 3 render them promising hosts for electron-rich planar aromatic compounds. Table 1 summarizes the association constants (Ka) of 2 and 3 with different aromatic compounds. Not surprisingly, there is virtually no association between p-dimethoxycyclohexane and either 2 or 3 and only a very small association between spherical sodium tetraphenylborate and 2 or 3. On the other hand, both 2 and 3 showed relatively strong association toward planar aromatic compounds. There are no clear binding differences between 2 and 3. The shape selectivity, however, deserves more attention and is being further investigated. A significant difference in Ka values was observed for the isomers of dimethoxybenzene, where m- and p-dimethoxybenzene >> o-dimethoxybenzene. A similar trend of shape selectivity has also been observed for Pd and Pt based square complexes.13
| Guest | 2 | 3 |
|---|---|---|
| a Binding was monitored by following the phenyl proton of TEPB for cage 2 and β proton of TPTA for cage 3. The spectra were recorded at 300 MHz in DMSO-d6 solution at 298 K. | ||
| o-Dimethoxybenzene | 0.81 × 102 | 0.99 × 102 |
| m-Dimethoxybenzene | 5.9 × 102 | 6.1 × 102 |
| p-Dimethoxybenzene | 4.5 × 102 | 4.2 × 102 |
| 1,3,5-Trimethoxybenzene | 8.9 × 102 | 9.1 × 102 |
| 2-Naphthalenesulfonic acid, sodium salt | 11.2 × 102 | 11.6 × 102 |
| 1,5-Naphthalenedisulfonic acid, disodium salt | 26.4 × 102 | 20.7 × 102 |
| 2,6-Naphthalenedisulfonic acid, disodium salt | 15.6 × 102 | 13.8 × 102 |
| Sodium tetraphenylborate | 0.75 × 102 | 0.60 × 102 |
| p-Dimethoxycyclohexane | <0.01 × 102 | <0.01 × 102 |
Acknowledgements
We are grateful to the U.S. Department of Energy (Grant DE-FG02-89ER14039) for support of this research.Notes and references
- J.-M. Lehn, Supramolecular Chemistry, VCH Publishers, New York, 1995. Search PubMed.
- S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 and references therein. Search PubMed.
- (a) (a) S.-S. Sun and A. J. Lees, J. Am. Chem. Soc., 2000, 122, 8956 CrossRef; (b) S.-S. Sun, A. S. Silva, I. M. Brinn and A. J. Lees, Inorg. Chem., 2000, 39, 1344 CrossRef CAS; (c) N. Takeda, K. Umemoto, K. Yamaguchi and M. Fujita, Nature, 1999, 398, 794 CrossRef CAS; (d) B. Olenyuk, M. D. Levin, J. A. Whiteford, J. E. Shield and P. J. Stang, J. Am. Chem. Soc., 1999, 121, 10434 CrossRef CAS; (e) S. M. Woessner, J. B. Helms, J. F. Houlis and B. P. Sullivan, Inorg. Chem., 1999, 38, 4380 CrossRef CAS.
- (a) (a) M. Fujita, N. Fujita, K. Ogura and K. Yamaguchi, Nature, 1999, 400, 52 CrossRef CAS; (b) B. Olenyuk, J. A. Whiteford, A. Fechtenkötter and P. J. Stang, Nature, 1999, 398, 796 CrossRef CAS; (c) M. Fujita, M. Aoyagi, F. Ibukuro, K. Ogura and K. Yamaguchi, J. Am. Chem. Soc., 1998, 120, 611 CrossRef CAS; (d) P. N. W. Baxter, J.-M. Lehn, B. O. Kneisel and D. Fenske, Angew. Chem., Int. Ed. Engl., 1997, 36, 1978 CrossRef CAS; (e) P. N. W. Baxter, J.-M. Lehn and A. DeCian, Angew. Chem., Int. Ed. Engl., 1993, 32, 69 CrossRef.
- (a) (a) K. D. Benkstein, J. T. Hupp and C. L. Stern, Angew. Chem., Int. Ed., 2000, 39, 2891 CrossRef; (b) T. Rajendran, B. Manimaran, F.-Y. Lee, G.-H. Lee, S.-M. Peng, C. M. Wang and K.-L. Lu, Inorg. Chem., 2000, 39, 2016 CrossRef CAS; (c) M. Schweiger, S. R. Seidel, M. Schmitz and P. J. Stang, Org. Lett., 2000, 2, 1255 CrossRef CAS; (d) K. D. Benkstein, J. T. Hupp and C. L. Stern, J. Am. Chem. Soc., 1998, 120, 12982 CrossRef CAS; (e) K. D. Benkstein and J. T. Hupp, Mol. Cryst. Liq. Cryst., 2000, 342, 151 Search PubMed.
- S.-S. Sun and A. J. Lees, Inorg. Chem., 1999, 38, 4181 CrossRef CAS.
- H. L. Anderson, C. J. Walter, A. Vidal-Ferran, R. A. Hay, P. A. Lowden and J. K. M. Sanders, J. Chem. Soc., Perkin Trans. 1, 1995, 2275 RSC.
- H. L. Anderson, S. Anderson and J. K. M. Sanders, J. Chem. Soc., Perkin Trans. 1, 1995, 2231 RSC.
- S.-S. Sun, E. Robson, N. Dunwoody, A. S. Silva, I. M. Brinn and A. J. Lees, Chem. Commun., 2000, 201 RSC.
- A. Vogler and J. Kisslinger, Inorg. Chim. Acta, 1986, 115, 193 CrossRef CAS.
- J. V. Caspar and T. J. Meyer, J. Phys. Chem., 1983, 87, 952 CrossRef CAS.
- CAChe Work System 3.8, Oxford Molecular, Oxford, 1995..
- M. Fujita, J. Yazaki and K. Ogura, Tetrahedron Lett., 1991, 32, 5589 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental procedures, characterization data for 2 and 3, a figure showing the ESI mass spectrum of cage 2 and a figure showing the electronic absorption spectra of 2 and 3 in MeCN solution. See http://www.rsc.org/suppdata/cc/b0/b007658i/ |
| ‡ After submission of our manuscript, an article describing the preparation of cages 2 and 3 using a similar two-step procedure appeared.5e |
| § Two possible geometrical isomers of 1 can exist, namely syn- and anti-isomers in terms of the mutual arrangement of two triflate ligands. The formation of subsequent cage compounds requires the presence of syn-isomers in solution. The high yields (>50%) of both cage-compounds suggests that either 1 existed mainly as the syn-isomer or a mixture of both syn and anti-isomers where the latter subsequently rearranges to the syn-isomer due to more favorable thermodynamic processes. |
| This journal is © The Royal Society of Chemistry 2001 |
