The allylic oxidation of unsaturated steroids by tert-butyl hydroperoxide using homogeneous and heterogeneous cobalt acetate†
Jorge A. R.
Salvador
and
James H.
Clark
Clean Technology Centre, Chemistry Department, University of York, York, UK YO10 5DD
First published on 11th December 2000
Abstract
Cobalt acetate is an effective catalyst for the selective allylic oxidation of unsaturated steroids using tert-butyl hydroperoxide especially when used in a supported form when it can be easily recovered and reused.
Allylic oxidation is a reaction of fundamental importance in organic chemistry with applications in areas ranging from agricultural products to pharmaceuticals.1,2
The allylic oxidation of unsaturated steroids such as Δ5-steroids has traditionally been carried out with chromium reagents such as CrO3–pyridine complex,3 chromium trioxide and 3,5-dimethylpyrazole,4 pyridinium chlorochromate, (PCC),5,6 pyridinium dichromate (PDC),6 sodium chromate,7 sodium dichromate in acetic acid8 and pyridinium fluoro- chromate.9 However the large excess of reagent used in these procedures along with a difficult work-up and production of environmentally hazardous chromium residues, makes these reactions increasingly unacceptable on a commercial scale. Of greater preparative interest has been the use of hydroperoxides combined with different types of catalysts.10–17 Despite the good yields reported with CrO3,10 hexacarbonylchromium Cr(CO)6,11,12 pyridinium dichromate13 and RuCl314 to prepare allylic oxidation products from Δ5-steroids, the toxicity of the chromium compounds and the high cost of the ruthenium catalyst renders the procedures unsuitable for commercialisation and led us to recently report the use of cuprous salts, CuBr, CuCl, Cul, and a cupric salt CuCl2, as well as copper metal as catalysts for this type of reaction.17
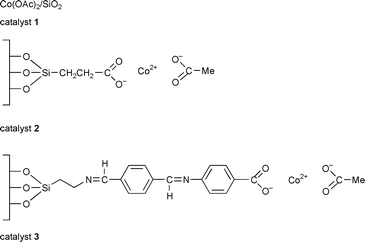
Allylic oxidation of steroids, particularly at the 7-position, has attracted interest over many years. The Δ5-steroids can be oxidised to 5-en-7-ones, which are known as inhibitors of sterol biosynthesis and have some use in cancer chemotherapy.18 This has encouraged us to find new, more environmentally acceptable methods for this reaction. The heterogenisation of inorganic reagents and catalysts useful in organic reactions is a very important area in clean technology.19 In this communication we report the use of cobalt acetate in homogeneous, (Co(OAc)2·4H2O), and more importantly, heterogeneous forms (catalysts 1, 2, and 3 prepared as reported previously20,21) for this type of allylic oxidation reaction.
Using Δ5-steroids 4–7 and 12 as substrates (Scheme 1) allylic oxidation products 8–11 and 13 were obtained in very high, isolated yields, 70–86% (Table 1). Apart from the reaction with substrate 6 which required benzene as solvent and a temperature of 70 °C all the reactions were performed in acetonitrile using a milder temperature of 50–55 °C. The best results were obtained using the supported catalyst 3 which may be a result of its greater organophilic character. No significant reaction occurs in the absence of catalyst or in the presence of the catalyst support only.
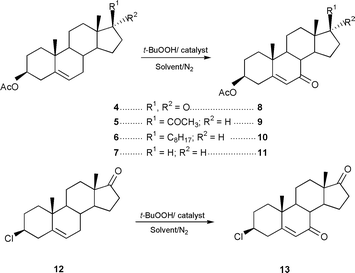 | ||
| Scheme 1 | ||
| Substrate/ mmol | t-BuOOHa/ml | Catalyst/mmol Co | Solvent | Time/h | Temp./°C | Prod. | Isolated yield (%) |
|---|---|---|---|---|---|---|---|
| a 5.0-6.0 M solution in decane (Aldrich). b Traces of starting material and a by-product are visible by TLC but not detectable in the 1H-NMR spectra (500 MHz) of the crude product. c Recovered by flash chromatography (ethyl acetate–petroleum ether 40–60 °C). d Calculated on the basis of the 1H-NMR signal (6H) of the crude product. | |||||||
| 4/1 | 1.2 | Co(OAc)2·4H2O/0.012 | CH3CN | 20 | 50 | 8 | 84b |
| 5/2 | 2.4 | Co(OAc)2·4H2O/0.024 | CH3CN | 24 | 50 | 9 | 86 |
| 4/1 | 1.2 | 1/0.01 | CH3CN | 18 | 50 | 8 | 85 |
| 4/1 | 1.2 | 2/0.009 | CH3CN | 20 | 50 | 8 | 84 |
| 4/1 | 1.2 | 2/(recycled, 0.008) | CH3CN | 20 | 50 | 8 | 80 |
| 4/1 | 1.2 | 1/ 0.005 | CH3CN | 24 | 50 | 8 | 40c |
| 6/1 | 1.2 | 2/0.022 | Benzene | 48 | 70 | 10 | 70c |
| 4/1 | 1.2 | 3/0.016 | CH3CN | 20 | 55 | 8 | 85 |
| 4/1 | 1.2 | 3/ (recycled, 0.014) | CH3CN | 20 | 55 | 8 | 81b |
| 4/2 | 2.4 | 3/0.0025 | CH3CN | 24 | 55 | 8 | 86 |
| 5/2 | 2.4 | 3/0.0025 | CH3CN | 20 | 50 | 9 | 82b |
| 7/1 | 1.2 | 3/ 0.007 | CH3CN | 24 | 55 | 11 | 72c |
| 12/0.65 | 0.8 | 3/0.016 | CH3CN | 20 | 55 | 13 | 73c |
| 14/0.63 | 0.8 | 3/0.006 | CH3CN | 3 | 55 | 15 | 70c |
| 16/1 | 1.2 | 3/0.016 | CH3CN | 24 | 55 | 17 | 71d |
In a typical reaction to a solution of 17-oxoandrost-5-en-3β-yl acetate 4 (660.90 mg 2 mmol) in acetonitrile (12 ml) under nitrogen, catalyst 3 (60 mg, cobalt loading 0.41 mmol g−1) and tert-butyl hydroperoxide (ca. 2.4 ml 12 mmol) were added. After 24 h under magnetic stirring at 50 °C, the catalyst was removed by filtration and the solution was poured into sodium sulphite solution (10% aq.) and extracted with diethyl ether. The extract was washed with aq. saturated solution of NaHCO3, water, dried and evaporated to dryness to give 7,17-dioxo- androst-5-en-3β-yl acetate. These reactions are very selective compared to those carried out using Fe(acac)3 as catalyst reported by Kimura et al.15,16 Mo(CO)6 has also been described as catalyst for this reaction, but this led to epoxidation of the cholesteryl acetate under similar oxidative conditions.16
While the product yields of the allylic oxidations are very similar under homogeneous and heterogeneous conditions, the easier recovery of the catalyst in the heterogeneous reactions make these more environmentally friendly processes. Furthermore using the heterogeneous catalysts 2 and 3 it was possible to reuse the catalyst with only a small reduction in the product yields, under similar experimental conditions (80% for recycled catalyst 2 and 81% for recycled catalyst 3, Table 1). Our catalytic method is also effective for other unsaturated steroids. Thus the Δ4-steroid 14 gives the testosterone acetate 15, in a yield of 70% (Scheme 2). Furthermore, the method is also effective in the presence of an oxidatively vulnerable secondary alcohol group. The steroid 16 is oxidised to the alcohol product 17 with impressive selectivity (71%) (Scheme 2).
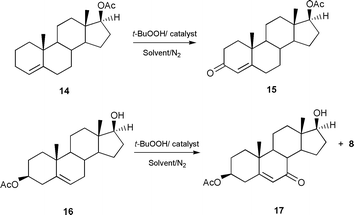 | ||
| Scheme 2 | ||
In summary we have discovered a new efficient and relatively environmentally friendly method for the preparation of Δ5-7-oxo-steroid and Δ4-3-oxo-steroid from easily available steroid substrates using t-BuOOH as the oxidant and supported Co(II) as an easily recoverable and reusable catalyst. A study of the effects of different oxidants and other types of heterogeneous catalyst on this and related reactions is presently under investigation.
Acknowledgements
We wish to thank the RAEng-EPSRC for a Clean Technology Fellowship (to J. H. C.) and the Universidade de Coimbra for financial support (to J. A. R. S.).Notes and references
- J. Muzart, Bull. Soc. Chim. Fr., 1986, 65, 2 Search PubMed.
- P. C. Bulman Page and T. J. Mccarthy, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Flemming, 1 Pergamon Press, Oxford, New York, Seoul, Tokyo, 1991, vol. 7, p. 83. Search PubMed.
- G. W. Dauben, M. Lorber and D. S. Fullerton, J. Org. Chem., 1969, 34, 3587 CrossRef; D. S. Fullerton and C. M. Chen, Synth. Commun., 1976, 6, 217 CAS.
- W. G. Salmond, M. A. Barta and J. L. Havens, J. Org. Chem., 1978, 43, 2057 CrossRef CAS.
- E. J. Parish, S. Chitrakorn and T.-Y. Wei, Synth. Commun., 1986, 16, 1371 CAS.
- E. J. Parish and T.-Y. Wei, Synth. Commun., 1987, 17, 1227 CAS.
- C. W. Marshall, R. E. Ray, I. Laos and B. Reigel, J. Am. Chem. Soc., 1957, 79, 6308 CrossRef CAS.
- A. Amann, G. Ourisson and B. Luu, Synthesis, 1987, 1002 CrossRef CAS.
- E. J. Parish, H. Sun and S. A. Kizito, J. Chem. Res., 1996, 544 Search PubMed.
- J. Muzart, Tetrahedron. Lett., 1987, 28, 4665 CrossRef CAS.
- A. J. Pearson, Y. S. Chen, Y. S. Hsu and T. Ray, Tetrahedron Lett., 1984, 25, 1235 CrossRef CAS.
- A. J. Pearson, Y. S. Chen, G. R. Hang, S. Y. Hsu and T. Ray, J. Chem. Soc., Perkin Trans. 1, 1985, 267 RSC.
- N. Chidambaram and S. Chandrasekaran, J. Org. Chem., 1987, 52, 5048 CrossRef CAS.
- R. A. Miller, W. Li and G. R. Humprey, Tetrahedron Lett., 1996, 37, 3429 CrossRef CAS.
- M. Kimura and T. Muto, Chem. Pharm. Bull., 1979, 27, 109 Search PubMed.
- M. Kimura and T. Muto, Chem. Phar. Bull., 1980, 28, 1836 Search PubMed.
- J. A. R. Salvador, M. L. Sá e Melo and A. S. Campos Neves, Tetrahedron Lett., 1997, 38, 119 CrossRef CAS.
- Y. Sato, Y. Sonoda, M. Morisaki and N. Ikekawa, Chem. Pharm. Bull., 1984, 32, 3305 Search PubMed; K. P. Cheng, H. Nagana, L. Bang and G. Ourisson, J. Chem. Res. (S), 1997, 217 Search PubMed; H. Nagana, J. P. Poyser, K. P. Cheng, L. Bang and G. Ourisson, J. Chem. Res. (S), 1997, 218 RSC; V. Kumar, A. Alain, G. Ourisson and B. Luu, Synth. Commun., 1987, 17, 1279 CAS.
- J. H. Clark, Catalysis of Organic Reactions using Supported Inorganic Reagents, VCH, New York, 1994 Search PubMed; Chemistry of Waste Minimisation, ed. J. H. Clark, Chapman and Hall, London, 1995. Search PubMed.
- A. J. Butterworth, J. H. Clark, P. H. Walton and S. J. Barlow, Chem. Commun., 1996, 1859 RSC.
- J. Chisem, I. C. Chisem, J. S. Rafelt, D. J. Macquarrie and J. H. Clark, Chem. Commun., 1997, 2203 RSC.
Footnote |
| † UK Patent applied for. |
| This journal is © The Royal Society of Chemistry 2001 |