Size and shape controlled ZnTe nanocrystals with quantum confinement effect
Young-wook Jun, Chang-Shik Choi and Jinwoo Cheon*
Department of Chemistry and School of Molecular
Science-BK21, Korea Advanced Institute of Science and Technology
(KAIST), Taejon, 305-701, Korea.. E-mail: jcheon@kaist.ac.kr
First published on 19th December 2000
Abstract
A simple one-pot synthesis of size and shape controlled ZnTe nanocrystals using a monomeric molecular precursor, [Zn(TePh)2][TMEDA], has been studied by varying the growth temperature or the templating surfactants.
In recent years, nanomaterials have drawn enormous interest from the scientific community because of their special characteristics that are different from the bulk such as quantum confined electronic band structures,1,2 novel optical,3 catalytic,4 and electronic properties.5 Since novel properties in nanoscale materials depend on their size and shape, one of the frontier issues of nanochemistry research is morphology controlled synthesis of nanocrystals.
While sulfur and selenium based II/VI nanocrystals such as CdS and CdSe are widely explored,6–9 there have not been many reports on the preparation of metal telluride nanocrystals. Furthermore, while there are some examples of Cd and Hg based tellurides,10–14 studies on ZnTe are very limited. ZnTe, an attractive semiconductor with a direct gap of 2.26 eV (ca. 548 nm) in the green region of the electromagnetic spectrum, can be a useful material in several applications such as green light-emitting diodes, buffer layers for HgCdTe IR detectors, or as the first unit in a tandem solar cell.15 Until now, only two reports on the preparation of colloidal ZnTe nanocrystals exist; however the resulting nanocrystals were either large (length 500–1200 nm, width 30–100 nm)16 or only their optical properties were investigated without isolation or characterization of the nanocrystals.17 In fact, there have not been any reports on isolated ZnTe nanocrystals <10 nm. When ZnTe particles are on the order of 10 nm, quantum size effects appear and control of the optical properties is possible by simply changing the particle size. It is then feasible to have tunability of the opto-electronic properties from the green to the UV region.
In this report, we describe a simple one-pot synthesis of morphology controlled ZnTe nanocrystals using a single molecular precursor, [Zn(TePh)2][TMEDA]. Upon thermolysis, this precursor produces ZnTe nanocrystals which are either spherical or of rod-like structure depending on the growth conditions and the choice of stabilizing surfactants. Furthermore, the size of the spherical ZnTe nanocrystals is controlled by the growth temperature and quantum confinement effects are observed. To the best of our knowledge, this paper reports the first isolated and well characterized ZnTe nanocrystals with quantum confined properties.
Even though metal chalcogenolato complexes have been widely studied previously as single-source precursors to group 12 chalcogenide semiconductor materials,6,12,18 the development of zinc tellurolate molecular chemistry is still limited.19–21 A pyridine adduct of a mesityltellurolate zinc complex prepared by Bochmann et al.22 and the dimeric compound [Zn{TeSi(SiMe3)3}2]2 prepared by Bonasia and Arnold23 have been known to generate ZnTe upon pyrolysis. In our study, we synthesized a polymeric phenyltellurolate zinc complex using a dealkylsilation process that was similarly employed to obtain a organotellurolato cadmium compound.24 The reaction of dimethylzinc with 2 equiv. of PhTeSiMe3 gives the pale yellow product Zn(TePh)2, which was then reacted with TMEDA to give [Zn(TePh)2][TMEDA].25 X-Ray crystallographic studies of [Zn(TePh)2][TMEDA]26 shows that it is monomeric and that the zinc center adopts a distorted tetrahedral geometry with a large Te–Zn–Te angle of 118.29(6)° and a small N–Zn–N angle of 84.1(4)° (Fig. 1).27
![ORTEP drawing of [Zn(TePh)2][TMEDA]. Selected bond distances
(Å) and angles (°): Te(1)–Zn(1) 2.5769(14),
Te(2)–Zn(1) 2.5876(15), Zn(1)–N(1) 2.126(9), Zn(1)–N(2)
2.145(9); Te(1)–Zn–Te(2) 118.29(6), N(1)–Zn(1)–N(2)
84.1(4).](/image/article/2001/CC/b008376n/b008376n-f1.gif) | ||
| Fig. 1 ORTEP drawing of [Zn(TePh)2][TMEDA]. Selected bond distances (Å) and angles (°): Te(1)–Zn(1) 2.5769(14), Te(2)–Zn(1) 2.5876(15), Zn(1)–N(1) 2.126(9), Zn(1)–N(2) 2.145(9); Te(1)–Zn–Te(2) 118.29(6), N(1)–Zn(1)–N(2) 84.1(4). | ||
A one-pot synthesis of ZnTe nanocrystals was carried out by the thermolysis of [Zn(TePh)2][TMEDA]. According to TGA, it is observed that the thermolysis of [Zn(TePh)2][TMEDA] begins with the dissociation of the TMEDA donor ligand, with ZnTe and Ph2Te produced in the following thermolysis step at higher temperatures.28 As similarly observed by Yamamoto and Steigerwald in related work on alkyl- or phenyl-chalcogenolate ligand systems,12,29 the thermolysis of its complexes cleanly produces the desired nanocrystals.
In a typical synthesis of spherical ZnTe nanocrystals, upon injection of the precursor (0.50 g, 0.88 mmol) dissolved in 5 ml of trioctylphosphine into the hot dodecylamine solvent (8.17 g, 44.1 mmol), immediate formation of nanocrystals was observed by the appearance of a pale yellow color. The crystal growth temperature was kept at either 180 or 240 °C for 2 h and the resulting solution was pale yellow in both cases. The solution was treated with butanol and centrifuged to isolate the yellow nanocrystals as a solid product.
Using the same conditions and procedures, the injection of the precursor into the mixed surfactant solvent trioctylamine (20 ml)–dimethylhexylamine (5 ml) and leads to a shape change of the nanocrystals from spherical to rod-like and after 2 h a gray precipitate of ZnTe nanocrystals was obtained from the initially pale yellow solution at 180 °C. It is believed that the combination of the two different surfactants provides rod-like micelles during the one-dimensional crystal growth process.30
The obtained spherical ZnTe nanocrystals are moderately monodispersed and their sizes can be controlled by changing the growth temperature. Relative to the position of the 548 nm (2.26 eV) absorption band edge of bulk ZnTe, blue shifts of 0.53 and 1.31 eV are found for the nanocrystals grown at 180 and 240 °C, respectively with larger shifts being seen for samples grown at higher growth temperature (Fig. 2A).31 Similar blue shifts are also observed in the photoluminescence spectra: band maxima are 451 and 377 nm, respectively, for samples grown at 180 and 240 °C (Fig. 2B). These results suggest that smaller nanocrystals are produced at higher growth temperatures where more nucleation sites exist and relatively less available ZnTe material is present for each nucleus during the growth process.
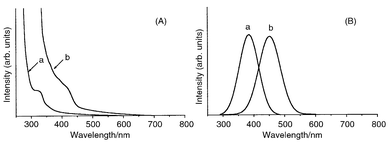 | ||
| Fig. 2 Optical spectra of ZnTe nanocrystals grown at (a) 240 and (b) 180 °C; (A) UV–VIS absorption spectra, (B) Photoluminescence spectra. | ||
High resolution transmission electron micrographs (HRTEM) show that the spherical ZnTe nanocrystals have average sizes of 4.2 (±1.1) and 5.4 (±0.9) nm for samples grown at 240 and 180 °C, respectively (Fig. 3A). Powder X-ray diffractometry (XRD) and selected area diffractometry (SAED) reveal patterns corresponding to (111), (220) and (311) of the cubic phase of ZnTe nanocrystals (Fig. 3C, D). This result is similar to that of TOPO-capped spherical ZnSe nanocrystals described in a previous report.31 TEM of the rod-like ZnTe nanocrystals show that the diameters of the rod-like nanocrystals are quite uniform (ca. 25 nm) with lengths of several hundred nanometers (200–700 nm) giving an aspect ratio from 8 to 30 (Fig. 3B). The rod-like ZnTe nanocrystals are also cubic phase as confirmed by XRD and SAED analysis.
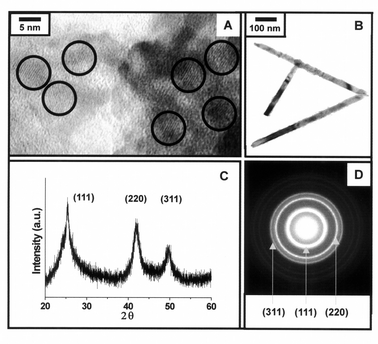 | ||
| Fig. 3 (A) HRTEM analysis of spherical ZnTe nanocrystals grown at 240 °C, (B) TEM analysis of rod-like ZnTe nanocrystals, (C) powder X-ray diffraction and (D) selected area diffraction patterns of 4.2 nm ZnTe nanocrystals. | ||
In conclusion, the results here constitute a simple and convenient one-pot synthesis of morphology controlled ZnTe nanocrystals using a monomeric molecular precursor, [Zn(TePh)2][TMEDA]. By varying the growth temperature or the choice of the templating surfactants, the size and shape of the nanocrystals are controllable and quantum size effects are observed. We believe that this strategy can be extended to the facile synthesis of nanocrystals of other materials.
Acknowledgements
This work was supported by the Tera Level Nanodevices National Program of KISTEP. We thank KBSI for the TEM analyses and Professor S. J. Kim and Dr Y.-M. Kim of Ewha Womans’ University for X-ray crystallographic analysis of the sample.Notes and references
- N. Chestnoy, R. Hull and L. E. Brus, J. Chem. Phys., 1986, 85, 2237 CrossRef CAS.
- L. E. Brus, J. Chem. Phys., 1984, 80, 4403 CrossRef CAS.
- G. Markovich, C. P. Collier, S. E. Henrichs, F. Remarcle, R. D. Levine and J. R. Heath, Acc. Chem. Res., 1999, 32, 415 CrossRef CAS.
- J. P. Wilcoxon, J. Phys. Chem. B, 2000, 104, 7334 CrossRef CAS.
- S.-H. Kim, G. Markovich, S. Rezvani, S. H. Choi, K. L. Wang and J. R. Heath, Appl. Phys. Lett., 1999, 74, 317 CrossRef CAS.
- See reviews: A. P. Alivisatos, J. Phys. Chem., 1996, 100, 13226 CrossRef CAS; A. Hagfeldt and M. Gratzel, Chem. Rev., 1995, 95, 49 CrossRef CAS; H. Weller, Angew. Chem., Int. Ed. Engl., 1993, 32, 41 CrossRef; J. R. Heath, Science, 1995, 270, 1315 CAS.
- T. Trindade and P. O’Brein, Chem. Mater., 1997, 9, 523 CrossRef CAS.
- X. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120, 5343 CrossRef CAS.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS.
- M. Mullenborn, R. F. Jarvis, B. G. Yacobi, R. B. Kaner, C. C. Colemann and N. M. Haegel, Appl. Phys. A, 1993, 56, 317 CrossRef.
- T. Rajh, O. I. Micic and A. J. Nozik, J. Phys. Chem., 1993, 97, 11999 CrossRef CAS.
- J. Brennan, T. Siegrist, P. J. Carroll, S. M. Stuczynski, P. Reynders, L. E. Brus and M. L. Steigerwald, Chem. Mater., 1990, 2, 403 CrossRef CAS.
- M. Gao, C. Lesser, S. Kirstein, H. Möhwald, A. L. Rogach and H. Weller, J. Appl. Phys., 2000, 87, 2297 CrossRef CAS.
- A. L. Rogach, A. L. Kershaw, M. Burt, M. Harrison, A. Kornowski, A. Eychmüller and H. Weller, Adv. Mater., 1999, 11, 552 CrossRef CAS.
- C. Bloomfield, Compd. Semicond., 1995, 1, 32 Search PubMed.
- Y. Li, Y. Ding and Z. Wang, Adv. Mater., 1999, 11, 847 CrossRef CAS.
- U. Resch, H. Weller and A. Henglein, Langmiur, 1989, 5, 1015 Search PubMed.
- M. Bochmann, Chem. Vap. Deposition, 1996, 2, 85 CAS; M. B. Hursthouse, M. A. Malik, M. Motevalli and P. O’Brein, Polyhedron, 1992, 11, 45 CrossRef CAS; B. O. Dabbousi, P. J. Bonasia and J. Arnold, J. Am. Chem. Soc., 1991, 113, 3186 CrossRef CAS; M. Bochmann, A. P. Colemann and A. K. Powell, Polyhedron, 1992, 11, 507 CrossRef CAS.
- L. Lange and W. W. Du Mont, J. Organomet. Chem., 1985, 286, C1 CrossRef CAS.
- P. J. Bonasia and J. Arnold, J. Chem. Soc., Chem. Commun., 1990, 1299 RSC.
- M. Bochmann and K. J. Webb, J. Chem. Soc., Dalton Tans., 1991, 2325 RSC.
- M. Bochmann, G. C. Bwembya, A. K. Powell and X. Song, Polyhedron, 1995, 14, 3495 CrossRef CAS.
- P. Bonasia and J. Arnold, Inorg. Chem., 1992, 31, 2508 CrossRef CAS.
- This compound was prepared according to a modification of the literature procedure: S. M. Stuczynski, J. G. Brennan and M. L. Steigerwald, Inorg. Chem., 1989, 28, 4431 CrossRef CAS.
- TMEDA (2.56 g, 22.0 mmol) was slowly added to Zn(TePh)2 (6.97 g, 14.7 mmol) in toluene (100 ml) and the reaction mixture was stirred for 24 h. After pyridine (10 ml) and heptane (50 ml) were added, insolubles were filtered off and the filtrate was concentrated and recrystallized at −24 °C to give colorless needle-shaped crystals. (6.02 g, 72.2%), mp 122–124 °C, Anal. Calc. for C18H26N2Te2Zn: C, 36.6; H, 4.40; N, 4.74; Te, 43.2; Zn, 11.1. Found: C, 36.6; H, 4.50; N, 4.70; Zn, 11.0%. δH(CDCl3,25 °C): 7.78 (d, 4H), 7.04 (t, 2H), 6.89 (t, 4H), 2.62 (s, 4H), 2.48 (s, 12H)..
- Crystal data: C18H26N2Te2Zn, Mr = 590.98, monoclinic, space group P21/n, a = 8.888(1), b = 17.866(3), c = 14.016(2) Å, β = 103.10(1)°, U = 2167.8(5) Å3, Z = 4, Dc = 1.811 g cm−3, F(000) = 1128, μ(Mo-Kα) = 3.772 mm, R1 = 0.0616, wR2 = 0.1659. CCDC 182/1854. See http://www.rsc.org/suppdata/cc/b0/b008376n/ for crystallographic files in .cif form..
- The Te–Zn–Te angles are slightly smaller than those reported for related compounds with larger ligands such as [Zn(mesityl)2(py)2] and [Zn{TeSi(SiMe3)3}2(py)2 ], (126.9 and 131.9°, respectively). The steric repulsions between these bulkier ligands are responsible for the larger angles as compared to compact phenyl ligands. This trend is clearly shown by the gradual increase of angle from 118.29 to 126.9 to 131.9° as the ligand size increases from phenyl to mesityl to sitel. The Zn–Te and Zn–N bond lengths are 2.5822(65) and 2.136(5) Å which are almost identical to those of [Zn{TeSi(SiMe3)3}2(py)2 ] and [Zn(mesityl)2(py)2] and similar to those seen for other zinc complexes with organochalcogen and amine ligands.19–21.
- In TGA, we observed that the dissociation of TMEDA and the generation of Ph2Te and ZnTe occur at 158 and 178 °C, respectively..
- K. Osakada and T. Yamamoto, J. Chem. Soc., Chem. Commun., 1987, 1117 RSC.
- M. P. Pileni, T. Gulik-Krzywicki, J. Tanori, A. Filankembo and J. C. Dedieu, Langmuir, 1998, 14, 7359 CrossRef CAS; Y. D. Li, H. W. Liao, Y. Ding, Y. T. Qian, L. Yang and G. E. Zhou, Chem. Mater., 1998, 10, 2301 CrossRef CAS; C. C. Chen, C. Y. Chao and Z. H. Lang, Chem. Mater., 2000, 12, 1516 CrossRef CAS.
- Y. Jun, J. Koo and J. Cheon, Chem. Commun., 2000, 1243 RSC.
| This journal is © The Royal Society of Chemistry 2001 |
