An alternative synthesis method for zeolite Y membranes
Guillaume
Clet†
*a,
Leszek
Gora
a,
Norikazu
Nishiyama‡
b,
Jacobus C.
Jansen
a,
Herman
van Bekkum
a and
Thomas
Maschmeyer
a
aLaboratory for Applied Organic Chemistry and Catalysis, Delft University of Technology, Julianalaan 136, 2628, BL, Delft, The Netherlands.. E-mail: guillaume.clet@ismra.fr
bLaboratory for Industrial Catalysis, Delft University of Technology, Julianalaan 136, 2628, BL, Delft, The Netherlands
First published on 11th December 2000
Abstract
A new approach for the synthesis of supported zeolite Y membranes is presented, using a synthesis gel containing seeds to avoid any unnecessary ex situ pre-treatment of the support.
Zeolitic membranes can be applied in many separations of either liquid or gaseous components. It is important to be able to draw from a large number of different zeolite membranes to enhance the scope of this approach further. Various procedures have been proposed for the synthesis of membranes with different zeolites, e.g. for MFI membranes a direct synthesis method has been reported1–3 as well as a synthesis route involving pre-adsorption of zeolite crystals and subsequent secondary growth.4,5 However, in some cases membranes become efficient only after many repeated syntheses steps.2 In another example vapour phase transport was used to synthesise mordenite and ferrierite membranes.6 Here we report a conceptually new and improved method for membrane preparation.
Owing to its large pore system and its specific counter-ion-dependent adsorption properties, zeolite Y is an interesting material for a membrane and/or a catalytic membrane reactor. Although zeolite Y membranes have been synthesised previously, the methods used involve the deposition of pre-formed crystals employed as seeds on the support7 or rubbing of the support with zeolite crystals.8,9 These ex situ synthesis methods work well on a laboratory scale, but are unlikely to be applicable for larger scale uses.
We showed recently that a coating of zeolite Y on common stainless steel could be achieved with a new synthesis procedure, using a seeded synthesis mixture.10 We report here that this method can be successfully employed to synthesise a zeolite Y membrane in situ. The behaviour of this material as a membrane was tested in the separation of the light molecules N2 and CO2, since membranes able to separate these technologically important gases are not common nor readily available.
Two different synthesis mixtures were prepared. The first mixture, prepared in two steps with sodium silicate as the silica source and sodium aluminate as the alumina source, was seeded according to the procedure described previously,10 by ageing a mixture highly concentrated in NaOH and incorporating it in a gel of general molar composition 10 SiO2∶Al2O3∶5.2 Na2O∶180 H2O (G1). A second type of gel, unseeded, was prepared in one step using colloidal silica (Ludox HS-40, DuPont) as the silica source with a general molar composition 10 SiO2∶Al2O3∶4 Na2O∶180 H2O (G2). Porous stainless steel disks, coated with a 15 μm thick porous titania layer (Trumem™) were used as supports. They were calcined at 300 °C prior to use to ensure they were free of organic deposits. Synthesis took place at 100 °C under rotational conditions (180 rpm) for 7 h in a Teflon-lined autoclave. During the synthesis, only the titania side was in contact with the synthesis mixture, the stainless steel side being protected by a Teflon disk. After synthesis the coated supports were washed and dried at 120 °C. Permeation measurements using single components of CO2 and N2 and binary mixtures of CO2/N2 were performed by the Wicke–Kallenbach method at 30 °C. The feed and permeate sides were kept at atmospheric pressure while helium was used as sweep gas with a flow rate of 100 ml min−1. Feed, retentate and permeate streams were analysed with a mass spectrometer. The zeolite layer on the support faced the feed side in the permeation measurements. Characterisation of the materials was performed by XRD and SEM. The Si/Al ratio was measured by ICP-AES of the powder and was equal to 2.
In agreement with previous observations on stainless steel plates,10 the use of the unseeded synthesis mixture (G2) resulted in a very low coverage of the titania layer even after 24 h of synthesis. Accordingly, no separation of the gas mixture could be observed. By contrast, when using the seeded mixture (G1), a good coverage of the support was obtained, although XRD could not evidence the presence of zeolite Y, probably owing to the thickness of the layer. However, the system did not show any real separation of N2 or CO2. When single component experiments were carried out, fluxes of N2 and CO2 were 8.58 × 10−7 and 6.05 × 10−7 mol m−2 s−1 Pa−1, respectively. The ideal selectivity and the selectivity measured for a 1∶1 mixture were nearly equal, at 0.7 and 0.63, respectively. Besides, large amounts of the sweep gas were found in the retentate. This indicates the presence of pinholes in the membrane. SEM showed that individual crystals were still present. This implies that crystal intergrowth was not achieved.
A second synthesis step can induce the intergrowth of the zeolite crystals supported after the first synthesis, however, when using the same seeded mixture (G1) for the second synthesis, degradation of the first layer occurred and impurities, namely zeolite P, were formed on the surface. This might be caused by the high pH of the synthesis mixture. To avoid the degradation of the zeolite Y layer, the second synthesis was performed, using the unseeded synthesis mixture (G2) of lower alkalinity. In this case, XRD evidenced that zeolite Y was present on the surface, and that impurities were not present. SEM images showed that the layer was well intergrown (Fig. 1). This layer was ca. 0.7 μm thick and permeation measurements confirmed that a closed layer had been synthesized.
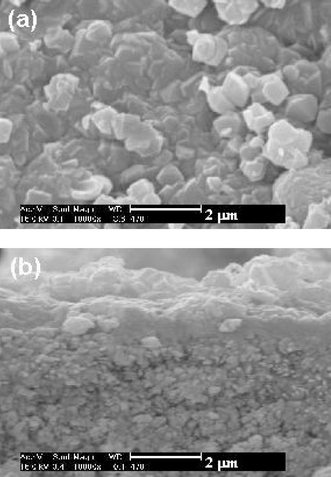 | ||
| Fig. 1 SEM images of the zeolite Y membrane on a TRUMEM support obtained sequentially after synthesis with G1 and G2 synthesis mixtures: (a) top view, (b) cross-section. | ||
Fig. 2 shows the fluxes of N2 and CO2 in the single- and binary-component measurements as a function of CO2 partial pressure for the latter material. Both curves cannot be explained by the Knudsen diffusion mechanism, since N2 diffuses faster than CO2 in the Knudsen diffusion region. The effect of the surface diffusion of CO2 should be taken into account. When plotting the separation factors of the CO2/N2 mixture vs. the CO2 partial pressure, the separation factor is greater than the ideal separation factor calculated with the single component fluxes. This indicates that adsorbed molecules of CO2 partly blocked the pores and depressed the permeation of N2. The separation factor CO2/N2 increased with increasing partial pressure of CO2, showing that the effect of pore blocking caused by adsorbed CO2 is more pronounced when the CO2 content in the feed stream is higher. This trend is in good agreement with the data obtained for the separation of other gas mixtures such as n-butane/methane,1n-butane/isobutane11 and ethane/methane12 over silicalite membranes. Although the separation behaviour was much improved, the fluxes did not decrease significantly (fluxes of N2 and CO2 were respectively 4.59 × 10−7 and 9.41 × 10−7 mol m−2 s−1 Pa−1 in single-component experiments). The presence of a dense amorphous phase is thus not likely as this would manifest itself in a lowering of fluxes. Furthermore, this interpretation is consistent with SEM characterisations (Fig. 1).
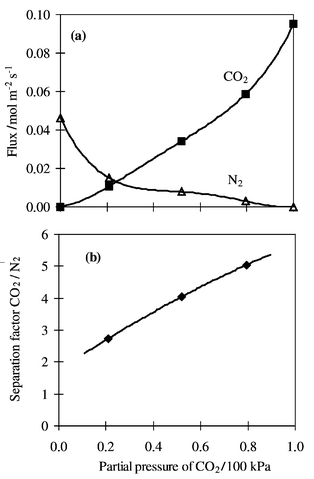 | ||
| Fig. 2 CO2/N2 permeation measurements. (a) fluxes, (b) separation factor (CO2/N2). | ||
We have shown that a high quality zeolite Y membrane can be synthesised by combining two synthesis steps. With this method a seeded gel layer is first deposited on the support upon dipping it in the synthesis mixture. The seeds initially present in the synthesis mixture are also part of the supported gel layer.13 Therefore their growth occurs directly on the support and this gives rise to a dense layer when exposing the system to a second synthesis step.
In conclusion, we have shown that seeds on the support are necessary to allow the growth of a supported Y membrane. Unlike previous reports,8,9 seeds can easily be brought to the support during synthesis. This method simplifies the process of attaching nuclei onto the support and opens up new vistas for supported zeolite membranes.
References
- E. R. Geus, H. van Bekkum, W. J. W. Bakker and J. A. Moulijn, Microporous Mater., 1993, 1, 131 CrossRef CAS.
- J. Coronas, J. L. Falconer and R. D. Noble, AIChE J., 1997, 43, 1797 Search PubMed.
- L. Gora, J. C. Jansen and Th. Maschmeyer, Stud. Surf. Sci. Catal., 1999, 125, 173 Search PubMed.
- M. C. Lovallo, A. Gouzinis and M. Tsapatsis, AIChE. J., 1998, 44, 1903 Search PubMed.
- R. Lai and G.R. Gavalas, Ind. Eng. Chem. Res., 1998, 37, 4275 CrossRef CAS.
- N. Nishiyama, K. Ueyama and M. Matsukata, AIChE J., 1996, 7, 299 Search PubMed.
- H. Kita, T. Inoue, H. Asamura, K. Tanaka and K. Okamoto, Chem. Commun., 1997, 45 RSC.
- K. Kusakabe, T. Kuroda, A. Murata and S. Morooka, Ind. Eng. Chem. Res., 1997, 36, 649 CrossRef CAS.
- I. Kumakiri, T. Yamaguchi and S.-I. Nakao, Ind. Eng. Chem. Res., 1999, 38, 4682 CrossRef CAS.
- G. Clet, J.C. Jansen and H. van Bekkum, Chem. Mater., 1999, 11, 1696 CrossRef CAS.
- L. P. J. van den Broeke, W. J. Bakker, F. Kapteijn and J. A. Moulijn, AIChE J., 1999, 45, 976 Search PubMed.
- J. M. van de Graaf, E. van der Bijl, A. Stol, F. Kapteijn and J. A. Moulijn, Ind. Eng. Chem. Res., 1998, 37, 4071 CrossRef CAS.
- G. Clet, J. A. Peters and H. van Bekkum, Langmuir, 2000, 16, 3993 CrossRef CAS.
Footnotes |
| † Present address: Laboratoire de Catalyse et Spectrochimie, ISMRa, University of Caen, 6, Bd du Maréchal Juin, 14050 Caen (cedex), France |
| ‡ Present address: Department of Chemical Engineering, Osaka University, 1-3 Machi-kaneyama, Toyonaka, Osaka 560-8531, Japan. |
| This journal is © The Royal Society of Chemistry 2001 |
