Dramatic acceleration of the catalytic process of the amination of allyl acetates in the presence of a tetraphosphine/palladium system
Marie Feuerstein, Dorothée Laurenti, Henri Doucet* and Maurice Santelli*
Laboratoire de Synthèse Organique UMR-CNRS 6009, Faculté des Sciences de Saint Jérôme, Avenue Escadrille Normandie-Niemen, 13397, Marseille, Cedex 20, France.. E-mail: henri.doucet@lso.u-3mrs.fr; m.santelli@lso.u-3mrs.fr
First published on 14th December 2000
Abstract
The cis,cis,cis-1,2,3,4-tetrakis(diphenylphosphinomethyl)-cyclopentan e/[PdCl(C3H5)]2 system catalyses allylic amination in good yields with a very high substrate/catalyst ratio; a turnover number of 680 000 and a turnover frequency of 8125 h−1 can be obtained for the addition of dipropylamine to allyl acetate in the presence of this catalyst.
Allylamines are fundamental building blocks in organic synthesis and their preparation is an important industrial goal.1 Allylic amination is an efficient method for the formation of allyl–nitrogen bonds.2 The classical method to perform this reaction is to employ palladium complexes associated with mono-3 or di-phosphine4 ligands. Phosphine-amine5 ligands have also been used successfully. Even if the catalysts formed by association of these ligands with palladium complexes are efficient in terms of yield of adduct, the efficiency in terms of the ratio of substrate/catalyst is low. In general 1–5% of these catalysts must be used. With one of the most active catalysts based on a polystyrene–phosphine–palladium complex the reaction can be performed with as little as 0.018% catalyst (TON: 3200).6 Nevertheless, very high values of ratio substrate/catalyst have not been reported for this reaction.
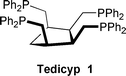
Our aim was to obtain complexes capable of very high turnover numbers in catalysis. The nature of the phosphine ligand on complexes has a tremendous influence on the rate of catalysed reactions. Recently a tetraphosphine based on a cyclobutane ring led to the formation of palladium catalysts for copolymerization that are more efficient than those of dppe by a factor of ten.7 In order to find more efficient palladium catalysts we decided to study the influence of the new tetrapodal phosphine ligand, cis,cis,cis-1,2,3,4-tetrakis(diphenylphosphinomethyl)cyclopentane (Tedicyp 1)8,9 in which the four diphenylphosphinoalkyl groups are stereospecifically bound to the same face of the cyclopentane ring, on the rate of allylic amination reaction.
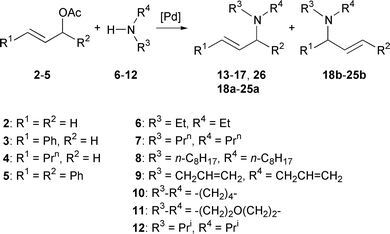
Our first objective was to evaluate the difference of efficiency for allylic amination between a monophosphine ligand such as triphenylphosphine, a diphosphine ligand such as 1,2-bis(diphenylphosphino)ethane (dppe) and our tetraphosphine 1. We observed that the addition of dioctylamine 8 to allyl acetate 2 in the presence of 0.001% catalyst, led to the addition product 14 in 1 and 3% conversion when PPh3 and dppe, respectively, were used as ligand and 99% conversion with Tedicyp (Scheme 1, Table 1). A similar tendency was observed for the addition of diallylamine 9 to allyl acetate 2. In the presence of 0.001% catalyst, only 7% conversion was observed with dppe. With Tedicyp the conversion was 73% in the presence of 0.0001% catalyst.
 | ||
| Scheme 1 | ||
| Allyl acetate | Amine | Product (major isomer) | Ratio a/bh | Ratio substrate/ catalyst | Turnover frequencyi/ h−1 | Yield (%) |
|---|---|---|---|---|---|---|
| Conditions: catalyst, see ref 10, THF, 25 °C, a 24 h.b 48 h.c 72 h.d 90 h.e 130 h.f 55 °C.g In toluene.h For compounds 18–25, a corresponds to the linear isomer and b to the branched isomer (Scheme 1). For compounds 29 and 30, a corresponds to the monoaddition product and b to the diaddition product (Scheme 2).i TOF calculated between initial time and 24 h.j Calculated after 48 h.k Calculated after 90 h. | ||||||
| 2 | 7 | 13 | — | 1000000 | 8125 | 68c |
| 5000000 | 8333 | 14ef | ||||
| 2 | 8 | 14 | — | 100000 | 2062j | 99bf |
| 1000000 | 7083 | 17af | ||||
| 2 | 9 | 15 | — | 100000 | 2388 | 95e |
| 1000000 | 8111k | 73df | ||||
| 2 | 11 | 16 | — | 10000 | 250 | 93e |
| 100000 | 1111 | 57e | ||||
| 2 | 12 | 17 | — | 1000 | 13 | 81c |
| 2 | 27 | 29a | 85/15 | 10000 | 250 | 97bf |
| 90/10 | 100000 | 1229 | 83cf | |||
| 2 | 28 | 30a | 68/32 | 1000 | 49 | 78ag |
| 3 | 7 | 18a | 94/6 | 1000 | 45 | 95a |
| 95/5 | 10000 | 160 | 36b | |||
| 3 | 9 | 19a | 95/5 | 1000 | 30 | 85e |
| 3 | 11 | 20a | 86/14 | 1000 | 41 | 99a |
| 84/16 | 10000 | 183 | 44a | |||
| 4 | 6 | 21a | 100/0 | 100 | 2.5 | 98e |
| 100/0 | 1000 | 6.6 | 43e | |||
| 4 | 7 | 22a | 100/0 | 1000 | 10.3 | 92d |
| 4 | 8 | 23a | 100/0 | 100 | 3.9 | 95a |
| 100/0 | 1000 | 13.3 | 84b | |||
| 4 | 10 | 24a | 93/7 | 100 | 4.1 | 98a |
| 94/6 | 1000 | 20.4 | 49bf | |||
| 4 | 11 | 25a | 91/9 | 100 | 4.1 | 100af |
| 92/8 | 1000 | 17.5 | 51e | |||
| 5 | 11 | 26 | — | 100 | 0.5 | 65ef |
Next we tried to evaluate the scope and limitations of Tedicyp–palladium complex for this reaction. The addition rate was slightly decreased for the addition of morpholine 11 to 2. A conversion of 93% is observed in the presence of 0.01% catalyst. A turnover number (TON) of 57 000 and a TOF of 1111 h−1 were obtained in the presence of 0.001% catalyst. On the other hand, a significant steric effect was observed with the bulky diisopropylamine 12. In the presence of 0.1% catalyst only 81% conversion was obtained after three days.
The complex formed by association of Tedicyp and [PdCl(C3H5)]2 seems to be more stable and less sensitive to temperature and poisoning than the complexes formed with diphosphines.
These results prompted us to investigate the allylation of amines with substituted allyl acetates. When we used cinnamyl acetate 3 in the presence of 0.1% catalyst high yields were obtained for the addition of dipropylamine 7 and diallylamine 9. A TON of 4400 has also been observed with morpholine 11. We noted a good regioselectivity for the amination of cinnamyl acetate 3 in favour of the linear isomer. Similar selectivities were observed for the addition of diethylamine 6, dipropylamine 7 and dioctylamine 8 to (E)-hex-2-en-1-yl acetate 4. (E)-N,N-dialkylhex-2-enylamines 21a–23a were obtained regio- and stereo-selectively in good yield. The regioselectivity of the addition of cyclic amines 10 and 11 is slightly lower; 6 and 8% of the branched products 24b and 25b are obtained with pyrrolidine 10 and morpholine 11. Much lower TON and TOF were observed in the course of the amination of hindered 3-acetoxy-1,3-diphenylprop-1-ene 5.
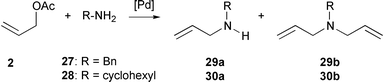
With primary amines, we obtained mixtures of monoaddition and diaddition products (Scheme 2). Benzylamine 27 led to the monoaddition product 29a in 83% conversion and 90% selectivity in the presence of 0.001% catalyst with a TOF of 1229 h–1. The addition rate of cyclohexylamine 28 is slower with a TOF of 49 h−1 and a lower selectivity in favour of the monoaddition product 30a is observed.
 | ||
| Scheme 2 Scheme 2 | ||
Finally, we tried to gain some information on the structure of the palladium–Tedicyp complex formed. Addition of 1 equiv. of Tedicyp to 0.5 equiv. of the dimer [PdCl(C3H5)]2 gave a clean 31P NMR spectrum which shows two broad signals at δ 19 and 25 (vs. H3PO4). The characteristic signals of the free phosphine at δ −16.3 and −17.7 were not observed. Addition of 1 equiv. of Tedicyp to 1 equiv. of [PdCl(C3H5)]2 gave an identical 31P NMR spectrum. Addition of 2 equiv. of Tedicyp to 0.5 equiv. of [PdCl(C3H5)]2 led to a more complicated spectrum; mainly four signals of free phosphines at δ −16.9, −18.2 , −19.3 and −20.9 and some broad peaks between δ 40 and 10 were observed in 31P NMR. In the first case, addition of 1 equiv. of Tedicyp to 0.5 equiv. of the Pd complex, produced broad signals at δ 19 and 25 suggesting that this complex is involved in a succession of equilibria due to a fast coordination–dissociation process of the four phosphines of the ligand. The absence of peaks of free phosphines probably arises from the equilibrium rate which seems to be of the order of the NMR time scale. Similar results have already been described; for example, Pd(PPh3)3 is largely dissociated and the equilibrium rate is of the order of the NMR time scale even at low temperature.12 Addition of 10 equiv. of allyl acetate or addition of 10 equiv. of allyl acetate with 10 equiv. of dipropylamine to this Pd–Tedicyp complex (ratio Pd-dimer/Tedicyp = 0.5) has no influence on the 31P NMR spectrum; the two broad signals observed at δ 19 and 25 are unchanged.
We have also examined the importance of the ratio palladium/Tedicyp for the catalysis. We observed that if the reaction is performed with Pd-dimer/Tedicyp ratios of 0.5, 1 and 2, the rate of the reaction decreases. The TONs after 40 min were, respectively, 8100, 4000 and 1300 for the addition of dipropylamine 7 to allylacetate 2 with a ratio substrate/catalyst of 100000. These results seems to indicate that the active palladium catalyst requires one tetraphosphine for one palladium centre.
In conclusion, the Tedicyp–palladium complex obtained by addition of Tedicyp to [Pd(C3H5)Cl]2 provides a convenient catalyst for the allylic amination reaction. This catalyst seems to be more stable and less sensitive to poisoning than the complexes formed with mono- and di-phosphine ligands. This stability probably arises from the presence of the four diphenylphosphinoalkyl groups stereospecifically bound to the same face of the cyclopentane ring. All four phosphines probably cannot bind at the same time to the same palladium centre, but the presence of these four phosphines on the ligand close to the metal centre, along with steric factors, seems to increase the coordination of the ligand to the palladium complex. In the presence of this catalyst the amination reaction can be performed with as little as 0.0001% catalyst. These results represent an inexpensive, efficient, and environmentally friendly synthesis. Further applications of this ligand will be reported in due course.
Acknowledgements
We thank F. Berthiol for experimental work and the CNRS for providing financial support.Notes and references
- For a review on allylic amination: see, M. Johannsen and K. Jorgensen, Chem. Rev., 1998, 98, 1689 CrossRef CAS.
- For reviews on palladium catalysed allylic substitution reactions see: T. Hayashi, in Catalytic Asymmetric Synthesis, ed. I. Ojima, VCH, New York, 1993, p. 325 Search PubMed; J. Tsuji, Palladium Reagent and Catalysis, Innovation in Organic Synthesis, Wiley, New York, 1995 Search PubMed; R. F. Heck, Palladium Reagents in Organic Syntheses, ed A. R. Katritzky, O. Meth-Cohn and C. W. Rees, Academic Press, London, 1985, p. 2 Search PubMed; A. Pfaltz and M. Lautens, Comprehensive Asymmetric Catalysis II, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, 1999, p. 833 Search PubMed; J.-L. Malleron, J.-C. Fiaud and J.-Y. Legros, Handbook of palladium catalyzed organic reaction, Academic Press, London, 1997. Search PubMed.
- N. Riegel, C. Darcel, O. Stéphan and S. Jugé, J. Organomet. Chem., 1998, 567, 219 CrossRef CAS; O. Kuhn and H. Mayr, Angew. Chem., Int. Ed., 1999, 38, 343 CrossRef CAS; J. P. Genêt, M. Balabane, J. E. Bäckvall and J. E. Nyström, Tetrahedron Lett., 1983, 24, 2745 CrossRef CAS; C. Thorey, J. Wilken, F. Hénin, J. Martens, T. Mehler and J. Muzart, Tetrahedron Lett., 1995, 36, 5527 CrossRef CAS; M. Moreno-Manas, L. Morral and R. Pleixats, J. Org. Chem., 1998, 63, 6160 CrossRef CAS.
- B. M. Trost, L. T. Calkins, C. Oerlet and J. Zambrano, Tetrahedron Lett., 1998, 39, 1713 CrossRef CAS; A. Yamazaki and K. Achiwa, Tetrahedron: Asymmetry, 1995, 6, 51 CrossRef CAS; N. Sirisoma and P. Woster, Tetrahedron Lett., 1998, 39, 1489 CrossRef CAS; Y. Uozumi, A. Tanahashi and T. Hayashi, J. Org. Chem., 1993, 58, 6826 CrossRef CAS.
- P. von Matt, O. Loiseleur, G. Koch, A. Pfaltz, C. Lefeber, T. Feucht and G. Helmchen, Tetrahedron: Asymmetry, 1994, 5, 573 CrossRef CAS.
- B. M. Trost and E. Keinan, J. Am. Chem. Soc., 1978, 100, 7779 CrossRef CAS.
- C. Bianchini, H. M. Lee, A. Meli, W. Oberhauser, F. Vizza, P. Brüggeller, R. Haid and C. Langes, Chem. Commun., 2000, 777 RSC.
- D. Laurenti, M. Feuerstein, G. Pèpe, H. Doucet and M. Santelli, unpublished results..
- cis,cis,cis-1,2,3,4-Tetrakis(diphenylphosphinomethyl)cycl opentane Tedicyp 1 was prepared from cis,cis,cis-1,2,3,4-tetrakis{[(tolyl-4- sulfonyl)oxy]methyl}cyclopentane 3113 by addition of Ph2PLi. 31P NMR of 1 (162 MHz, THF-d8) δ −16.3, −17.7..
- The cis,cis,cis-1,2,3,4-tetrakis(diphenylphosphinomethyl)cyclopentane /[PdCl(C3H5)]2 complex was prepared by stirring under argon the tetraphosphine 1 (140 mg, 162 μmol) with [PdCl(C3H5)]2 (30 mg, 81 μmol) in THF (10 ml) for 10 min at room temperature. This complex is not air stable and must be prepared just before use. δP(162 MHz, CDCl3) 25 (w = 80 Hz), 19.4 (w = 110 Hz)..
- In a typical experiment, the reaction of cinnamyl acetate 3 (1.30 g, 7.4 mmol) and dipropylamine 7 (2.07 g, 14.8 mmol) at 50 °C for 90 h in distilled THF (20 mL) in the presence of the Tedicyp–palladium complex (7.4 × 10−3 mmol) under argon affords the corresponding addition product 18 after evaporation and filtration on silica gel (diethyl ether–pentane: 3/7) in 95% (1.52 g) isolated yield..
- C. Amatore, A. Jutand, F. Khalil, M. M’Barki and L. Mottier, Organometallics, 1993, 12, 3168 CrossRef CAS; C. Amatore, G. Broeker, A. Jutand and F. Khalil, J. Am. Chem. Soc., 1997, 119, 5176 CrossRef CAS.
- K. Somekawa, M. Nomura, S. Aoki, S. Noma and T. Shimo, Org. Prep. Proc. Int., 1993, 25, 449 Search PubMed.
| This journal is © The Royal Society of Chemistry 2001 |