Development of a light-responsive permeation membrane modified by an azo derivative on a porous glass substrate
Tetsuro
Jin
*,
Aliyar H.
Ali
and
Tetsuo
Yazawa
Department of Optical Materials, Osaka National Research Institute, AIST, MITI,1-8-31 Midorigaoka, Ikeda, Osaka, 563-8577, Japan.. E-mail: tetsujin@onri.go.jp
First published on 15th December 2000
Abstract
A photo-responsive gas separation membrane modified by an organic azo derivative in a porous glass tube shows decreased gas permeance upon stimulation with an Xe- lamp, which returns the starting level upon stopping the irradiation.
It is well known that azobenzene derivatives show photo-reversible cis–trans isomerization.1,2 Such conformational change leads to many chemically photoinduced changes so that many applications can be envisaged; among these is the possibility to control the chemical functions by ‘on–off switching’. Several investigations considering this concept have been reported.3–5 In addition, there are many researchers that are attempting to develop membranes with stimuli–response functions. For example, polymeric separation membranes with stimulation–response functions such as pH ultrafiltration membranes7,8 or liquid membrane separating the intended ions by light9 are now actively investigated.
On the other hand, porous glass membranes which possess relatively sharp pore size distributions are now actively investigated because of their high gas selectivity, ease of control of the pore size and easier molding.6 However, the pore size of all such membranes is fixed rigidly so no such membranes can respond to physical and/or chemical stimuli.
In this study, as a new concept, the preparation of a porous glass membrane surface modified by an azo derivative was carried out for use of gas flow control. Furthermore, control of the gas permeability by the photoinduced cis–trans switching property of the compound under light irradiation was investigated.
Porous glass tubes were prepared and gas permeance measurements at 300 K were carried out according to the procedure reported elsewhere.10 The chemicals which were used in this study were purchased either from Wako Pure Chemical Industries Co., Ltd. and Aldrich Inc. or used without any purification. The azo derivative, 11-[4-[(4′-hexylphenyl)- azo]phenoxy]undecanoic acid (6Az10CO2H) was synthesized using the route reported by Seki et al.11 Initially, a porous glass surface treated with 3-aminopropyldimethylethoxysilane as a coupling agent was placed into a solution of 6Az10CO2H and THF and refluxed at 373 K for 20 h. Subsequently, the porous glass was refluxed in THF for >24 h and then washed with THF several times. The azo modification membrane tube (one end was sealed by epoxy resin) was attached to the Pyrex glass tube by epoxy resin and then the support tube with the membrane was placed inside a stainless steel permeation cell equipped with a silica window through a flange connection, after drying at 348 K in vacuum for 1 h. Using the prepared membrane, gas permeances of pure N2 and He were measured by a mass flow meter. The single feed gas of N2 and He was passed continuously outside of the membrane under a pressure of ca. 3.03 × 105 Pa (3.0 atm) at room temperature (300 K). Light irradiation from a Xe lamp (500 mW cm−2) was directed to the quartz window which could be covered with a thick board to interrupt irradiation. The pore size distribution of the sample after drying at 348 K under vacuum for several hours was measured by a nitrogen adsorption isotherm using a conventional volumetric apparatus (BELSORP 28SA, BEL JAPAN, inc.). Absorption spectra were recorded on a Shimadzu model 2501 instrument controlled by a personal computer.
The pore size distributions of the porous glass membranes before and after modification with the azo derivative were measured by nitrogen adsorption isotherms. As shown in Fig. 1, a main peak at ca. 1.7 nm (radius) for the non-modified and azo modified glass tubes was observed. The surface area of the azo modified glass tube (surface area: 108.9 m2 g−1, pore volume: 9.10 × 10−4 m3 g−1) was ca. 30% lower than that of the non-treated sample (surface area: 152.0 m2 g−1, pore volume: 1.36 × 10−3 m3 g−1). This suggested that 6Az10CO2H is mainly located on the surface of the porous glass and not in the pores.
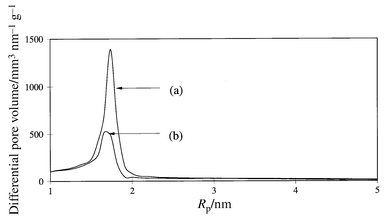 | ||
| Fig. 1 Pore size distribution for the porous glass tube (a) before and (b) after surface modification with 6Az10CO2H. | ||
By using the modified membrane, changes of N2 flow before and
after Xe-lamp irradiation were investigated at 300 K. As shown in Fig. 2, the flow of each gas drastically decreased
during Xe-lamp irradiation while the flow increased by stopping
irradiation. These cycles could be continued >30 times. Furthermore,
there was no light response when similar experiments were performed using
non-treated porous glass or porous glass treated with the silane coupling
agent only. The increase in temperature close to the modified membrane in
the apparatus upon Xe-lamp irradiation was 3–5 K. Thus thermal
vibrations around the pores of the glass tube gradually increased the
amplitude of N2 permeance. The length of the folded
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) NC6H4C6H13 moiety of
the modifier is 2.377 nm and from these results reorientation of this part
of the modifier upon isomerization would reduce the gas permeance by
covering the entrance of the pores of the glass substrate.
NC6H4C6H13 moiety of
the modifier is 2.377 nm and from these results reorientation of this part
of the modifier upon isomerization would reduce the gas permeance by
covering the entrance of the pores of the glass substrate.
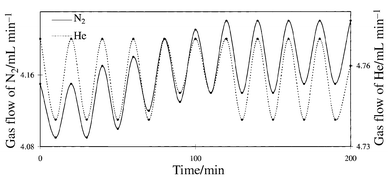 | ||
| Fig. 2 Gas flow variation of N2 (——) and He (- - - -) passing through the surface modified glass membrane under Xe-lamp irradiation or non-irradiation | ||
The absorption spectrum of the azo modified sample under Xe-lamp irradiation is plotted in Fig. 3 which shows a peak at ca. 360 nm from the trans form and a broad peak at ca. 480 nm from the cis form with a trans/cis ratio of 90/10. On the other hand, this ratio was reduced to 75/25 when UV light (low pressure Hg lamp) was used as a light source and no light response to gas flow was observed. Variation in the permeance relates to rapid cis to trans isomerization with the cis form of the derivative being resistant to permeance (see results of pore size distribution), however, a detailed mechanism could not be established in this study.
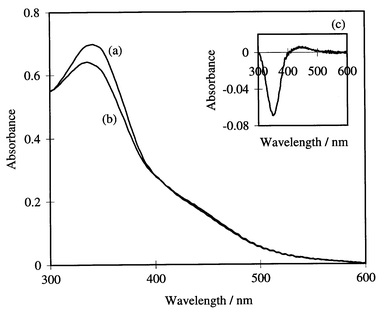 | ||
| Fig. 3 Absorption spectra of the surface modified glass membrane (a) before irradiation, (b) under Xe-lamp irradiation and (c) differential spectrum (b) − (a). | ||
Thus, further experiments are now being performed to establish the detailed mechanism of the permeation in an attempt to obtain finer regulation for several gases by considering the interplay between the molecular size of the azo derivative and the pore size of the substrate.
Acknowledgements
We wish to acknowledge Dr K. Tawa for her assistance in measuring the UV–VIS spectra of the sample. We also thank Mrs H. Kobayashi and Y. Tatematsu for their help on every experiment in this study.Notes and references
- D. L. Beveridge and H. H. Jaffe, J. Am. Chem. Soc., 1966, 88, 1948 CrossRef CAS.
- S. Llunggren and G. Wettermark, Acta Chem. Scand., 1971, 25, 1599 Search PubMed.
- S. Shinkai, T. Ogawa, T. Nakaji, Y. Kusano and O. Nanabe, Tetrahedron Lett., 1979, 4569 CrossRef CAS.
- H. S. Blair and C. B. McArdle, Polymer, 1984, 25, 999 CrossRef CAS.
- T. Seki, R. Fukuda, M. Yokoi, T. Tamaki and K. Ichikawa, Bull. Chem. Soc. Jpn., 1996, 69, 2375 CAS.
- T. Yazawa, Membrane, 1995, 20, 183 Search PubMed.
- A. L. Nguyen and J. H. T. Luong, Biotechnol. Bioeng., 1989, 34, 1186 CrossRef CAS.
- M. Taniguchi, M. Kobayashi, K. Natsumi and M. Fujii, J. Ferment. Bioeng., 1989, 68, 32 Search PubMed.
- S. Shinkai, T. Ogawa, T. Nakaji and O. Manabe, J. Chem. Soc., Chem. Commun., 1980, 375 Search PubMed.
- T. Yazawa and H. Tanaka, Ceram. Trans., 1993, 31, 213 Search PubMed.
- T. Seki, M. Sakuragi, Y. Kawanishi, Y. Suzuki, T. Tamaki, R. Fukuda and K. Ichimura, Langmuir, 1993, 9, 211 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |
