A novel Lewis acid-promoted enyne cycloisomerization of triester-substituted alkenes†
Shoko
Yamazaki
*a,
Kuriko
Yamada
a,
Tetsuya
Otsubo
a,
Masayo
Haruna
a,
Emiko
Kutsuwa
a and
Hatsue
Tamura
b
aDepartment of Chemistry, Nara University of Education, Takabatake-cho, Nara, 630-8528, Japan.. E-mail: yamazaks@nara-edu.ac.jp
bDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 1-16 Machikaneyama, Toyonaka, Osaka, 560-0043, Japan
First published on 15th December 2000
Abstract
A novel cycloisomerization reaction of enynes 4 in the presence of ZnBr2 and THF (1 eq.) in CH2Cl2 at −40 °C gave exo-methylenic 1,3-dienes 5 in moderate to good yield.
Lewis acid-promoted reactions are an important part of modern synthetic chemistry.1 Recently, prototropic cycloisomerization reactions of enynes and dienes have been recognized as atom-economic tools to construct rings, and transition metal-catalyzed cycloisomerizations in particular have received much attention.2 However, only a few examples of Lewis acid-promoted cycloisomerizations (intramolecular ene reactions) have been reported so far.3,4a,b
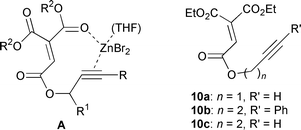
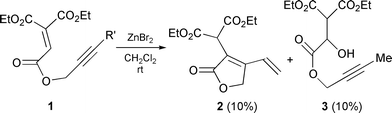
The design of a Lewis acid-promoted enyne cyclization requires that a highly electrophilic alkene (or alkyne) has coordination sites for a Lewis acid and that the other component (alkyne or alkene) works as a nucleophile. Alkenes with three ester groups are considered to be highly electrophilic and very reactive towards nucleophilic olefins,5 and they may react intramolecularly with alkynes which are relatively unreactive nucleophiles.6‡ We thus examined Lewis acid-promoted intramolecular cyclization of the designed triester-substituted alkene 1. Reaction of 1 in the presence of ZnBr2 at rt gave cyclized product 2 and H2O-adduct 3 in 45% and 10% yields, respectively (eqn. (1)).
 | (1) |
The formation of 2 can be explained as shown in Scheme 1.§ Nucleophilic attack of the alkyne moiety to the electrophilic olefin complexed with ZnBr2 in A gives intermediate B.¶ A 1,3-hydride shift then leads to complex C, which isomerizes and then forms diene 2 with Et3N. The formation of hydrated product 3 is presumed to result from attack by trace amounts of water on complex A.
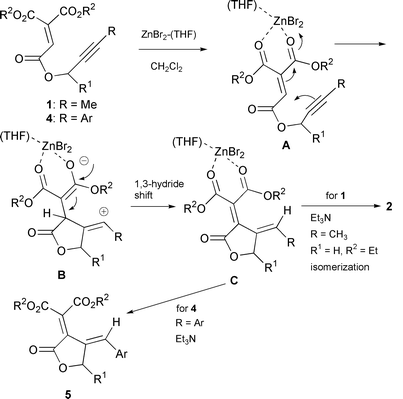 | ||
| Scheme 1 Scheme 1 | ||
If the isomerization step in C is prevented,
exo-methylenic 1,3-dienes should be the primary products in this
process. The enyne 4, which is prevented from undergoing
isomerization due to the lack of a proton, was designed and Lewis acid
mediated reactions examined. After several conditions were examined (see
below), reaction of 4 in the presence of ZnBr2 (1.2
eq.) and THF (1 eq.) in CH2Cl2 at −40 °C
for 17–19 h gave exo-methylenic 1,3-dienes 5 in
29–67% yields (eqn. (2), Table 1).||
The γ-lactone structure of 5 was suggested by the presence
of a characteristic C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O absorption (1771–1779
cm−1). 1H, 13C,
1H/13C-HSQC, HMBC and NOESY spectra were in agreement
with the lactone structure drawn in eqn.
(2). The crystal structure of 5a was elucidated by X-ray
diffraction analysis (Fig. 1).**
The diene is slightly twisted from the plane in order to reduce steric
repulsion between the ester and HPhC
O absorption (1771–1779
cm−1). 1H, 13C,
1H/13C-HSQC, HMBC and NOESY spectra were in agreement
with the lactone structure drawn in eqn.
(2). The crystal structure of 5a was elucidated by X-ray
diffraction analysis (Fig. 1).**
The diene is slightly twisted from the plane in order to reduce steric
repulsion between the ester and HPhC![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) groups; the torsion angle of
the diene moiety (∠C5-C3-C2-C12) is 21.9°. This cisoid
diene may be effective as an acceptor in the inverse electron demand
Diels–Alder reaction (see below).
groups; the torsion angle of
the diene moiety (∠C5-C3-C2-C12) is 21.9°. This cisoid
diene may be effective as an acceptor in the inverse electron demand
Diels–Alder reaction (see below).
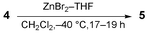 | (2) |
| Substratea | R1 | R2 | Ar | Product (yield) |
|---|---|---|---|---|
| a All reactions were carried out using 0.30–0.58 mmol of 4, 1.2 eq. of ZnBr2, and 1.0 eq. of THF at 0.41 M for 4 in CH2Cl2 at −40 °C for 17–19 h, unless otherwise noted. b THF was not added. Addition of THF gave 5g in 40% yield along with recovered 4g (24%). | ||||
| 4a | H | Et | Ph | 5a (67%) |
| 4b | H | Et | p-Tol | 5b (58%) |
| 4c | H | Et | p-MeO-C6H4 | 5c (41%) |
| 4d | Me | Et | Ph | 5d (53%) |
| 4e | n-Propyl | Et | Ph | 5e (46%) (4e recovered 36%) |
| 4f | H | Me | Ph | 5f (29%) |
| 4g | H | iPr | Ph | 5g (50%)b |
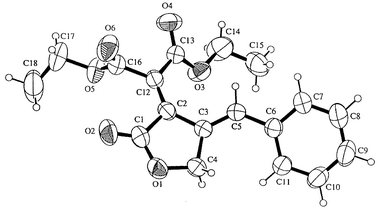 | ||
| Fig. 1 ORTEP drawing of 5a (50% probability ellipsoids). Selected bond lengths (Å) and torsion angle (°): C1–C2 = 1.499(3); C2–C3 = 1.460(2); C3–C4 = 1.501(2); C1–O1 = 1.344(2); O1–C4 = 1.444(2); C2–C12 = 1.345(2); C3–C5 = 1.344(2); ∠C5–C3-C2–C12 = 21.9(3). More detailed structure data are given in the supplementary data. | ||
With 4a,b, the yield of 5 decreased when THF was omitted from the reaction (for 5a to 11–36%, for 5b to 11–25%).†† The effect of THF is presumed to be that coordination of THF to Zn adjusts the strength of the Lewis acid and prevents side reactions.‡‡ For 4g, the reaction without THF gave a slightly better yield (see Table 1).
Use of ZnI2–THF instead of ZnBr2–THF gave 5a in 62% yield. Use of SnCl4 (−78 °C) or ZnCl2–THF (−40 °C) gave 5a in lower yield (24–-39% including inseparable complex mixtures). The reaction of 4a with ZnBr2–THF was also performed at a higher temperature (0 °C to rt), however, the yield of 5a decreased (32%), probably because of the instability of the diene product. The reaction of 4a using 0.3 eq. of ZnBr2–THF afforded 19% of 5a along with recovered 4a (62%), therefore the reaction requires a stoichiometric amount of Lewis acid.§§
Thermal reactions of 4a and 1 (CH3CN, 80 °C, 24 h or toluene, 110 °C, 24 h) without Lewis acid only afforded complex mixtures along with recovered starting materials (38–87%). A RuClH(CO)(PPh3)3-catalyzed reaction (toluene, 110 °C, 7 h) of 4a was examined but did not proceed.8
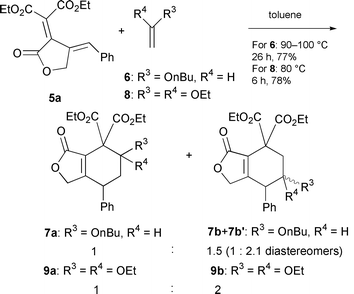
Only a few examples of synthesis of heterocycles by cycloisomerization using transition metal catalysts have been reported.9 Therefore, the present method should provide an efficient alternative to transition metal-catalyzed cycloisomerizations. Also, the product cyclic dienes are electron-deficient and suitable for cycloadditions such as inverse electron demand Diels–Alder reaction10 and transition metal-catalyzed [4 + 3] cycloadditions.11 The inverse electron demand Diels–Alder reactions of 5a with the electron rich dienophiles 6 and 8 were thus examined (eqn. (3)). C–C bond formation proceeds readily, however, the observed regio- and stereoselectivity was low.¶¶
 | (3) |
In summary, a novel Lewis acid-promoted enyne cycloisomerization to give cyclic dienes has been developed. This new reaction provides a highly efficient means to prepare electron-deficient cyclic dienes. The application of this methodology towards the construction of carbocycles and diverse heterocycles is under investigation.
Acknowledgements
We are grateful to Dr K. Yamamoto (Osaka University) for measurement of mass spectra and elemental analysis. We thank Mr Y. Yanase for experimental assistance.Notes and references
- Selectivities in Lewis Acid Promoted Reactions, ed. D. Schinzer, Kluwer Academic Publishers, Dordrecht, 1989 Search PubMed; S. Shambayati and S. L. Schreiber, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, Vol. 1, p. 283. Search PubMed.
- B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS.
- B. B. Snider, Acc. Chem. Res., 1980, 13, 426 CrossRef CAS; K. Mikami and M. Shimizu, Chem. Rev., 1992, 92, 1021 CrossRef CAS.
- (a) K. Narasaka, Y. Hayashi and S. Shimada, Chem. Commn., 1988, 1609 Search PubMed; (b) T. Minami, T. Utsunomiya, S. Nakamura, M. Okubo, N. Kitamura, Y. Okada and J. Ichikawa, J. Org. Chem., 1994, 59, 6717 CrossRef CAS.
- W. Srisiri, A. B. Padias and H. K. Hall, Jr., J. Org. Chem., 1994, 59, 5424 CrossRef CAS.
- (a) T. R. Kelly, Tetrahedron Lett., 1973, 437 CrossRef CAS; (b) B. B. Snider, D. M. Roush and T. A. Killinger, J. Am. Chem. Soc., 1979, 101, 6023 CrossRef CAS; (c) B. B. Snider and D. M. Roush, J. Org. Chem., 1979, 44, 4229 CrossRef CAS.
- I. Suzuki and Y. Yamamoto, J. Org. Chem., 1993, 58, 4783 CrossRef CAS.
- M. Nishida, N. Adachi, K. Onozuka, H. Matsumura and M. Mori, J. Org. Chem., 1998, 63, 9158 CrossRef CAS.
- B. M. Trost, E. D. Edstrom and M. B. Carter-Petillo, J. Org. Chem., 1989, 54, 4489 CrossRef CAS.
- G. J. Bodwell and Z. Pi, Tetrahedron Lett., 1997, 38, 309 CrossRef CAS; H. L. Gingrich, D. M. Roush and W. A. Van Saun, J. Org. Chem., 1983, 48, 4869 CrossRef CAS.
- B. M. Trost and D. T. Macpherson, J. Am. Chem. Soc., 1987, 109, 3483 CrossRef CAS.
- N. Asao, T. Asano, T. Ohishi and Y. Yamamoto, J. Am. Chem. Soc., 2000, 122, 4817 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental procedures and spectral data for described compounds and crystallographic data for 5a. See http://www.rsc.org/suppdata/cc/b0/b008103p/ |
| ‡ Thermal ene reactions6a,b and FeCl3-promoted chlorinated cyclizations6c of allylic and propargylic esters of ethylenetricarboxylic acid have been reported. |
| § The alternative mechanism is that 1 undergoes an ene reaction initially to give a cyclic allene that rearranges to 2. |
¶ The
coordination of a Lewis acid to a C![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) C bond was reported
recently.12 The intermediate A
(Scheme 1) can be drawn as shown
above. C bond was reported
recently.12 The intermediate A
(Scheme 1) can be drawn as shown
above. |
| || Cyclizations were also examined using 10a–c as substrates. Using similar conditions, only starting material was recovered. |
| ** Crystal data: C18H18O6, M = 330.34, monoclinic, a = 8.1206(3), b = 10.5914(3), c = 19.7984(7) Å, β = 100.513(1)°, V = 1674.2(1) Å3, T = 296 K, space group P21/c (no. 14), Z = 4, μ(Mo-Kα) = 0.099 mm–1, number of reflections measured = 4062, number of independent reflections = 3845 (Rint = 0.025), R, Rw = 0.047, 0.052 for 2473 observed reflections (I > 2σ(I)). CCDC 182/1839. See http://www.rsc.org/suppdata/cc/b0/b008103p/ for crystallographic files in .cif format. |
| †† The reaction of 4a in THF as a solvent did not proceed. |
| ‡‡ Use of propylene oxide instead of THF in the reaction of 4a gave 5a in lower yield (28%), along with recovered 4a (21%). The combination of Lewis acid and Lewis base is used in some Lewis acid-mediated reactions.7 |
| §§ Formation of cyclic dienes is in marked contrast with the FeCl3-promoted reaction of dimethyl ester analog of 1 and 4d giving chlorinated cyclization products.6c Investigation of the difference in Lewis acids is underway. |
| ¶¶ The stereochemistries of 7a, 7b and 7b′ were tentatively assigned as shown in the supplementary information by the observed NOE’s. |
| This journal is © The Royal Society of Chemistry 2001 |