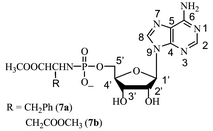
Reaction of ADP with amino acid methyl esters mediated by trimethylsilyl chloride
Hua
Fu
*,
Bo
Han
and
Yu-Fen
Zhao
Bioorganic Phosphorus Chemistry Lab of Education Ministry, Department of Chemistry, School of Life Sciences and Engineering, Tsinghua University, Beijing, 100084, P. R. China. E-mail: fuhua@mail.tsinghua.edu.cn; Fax: 86 10 6278 1695; Tel: 86 10 6277 2259
First published on 2nd December 2002
Abstract
Reaction of ADP with amino acid methyl esters mediated by trimethylsilyl chloride in pyridine produced adenosine 5′-phosphoramidates in good yields under mild conditions, it is interesting that nucleophilic attack of amino acid methyl esters only occurred on α-phosphorus of ADP.
In biological systems, reactions of polyphosphates, for example ATP and GTP, with nucleophiles such as water, alcohols and amines, are usually observed. One of the most important examples is nucleotidyl transfer in RNA capping reactions1–3 and DNA ligation reactions.4,5 The reaction of ATP or GTP with ε-NH2 of lysine residues in enzymes produces covalent enzyme–NMP (nucleoside 5′-monophosphate) intermediates containing a P–N bond in the presence of Mg2+ under physiological environments.6 Subsequent nucleophilic attack of 5′ or 3′-hydroxyl of RNA or DNA to the enzymatic intermediates leads to decomposition of the enzymes and elongation of the RNA chain or ligation of DNA strands. On the other hand, medicinal chemists have also made use of amino acid decomposition of nucleoside 5′-phosphoramidates in biological systems to develop a series of prodrugs, and found that these nucleoside derivatives showed potent anticancer, antiviral and anti-HIV activity and low cytotoxicity compared with their parent nucleosides.7–9 The reported synthesis of nucleoside 5′-phosphoramidates include DCC-coupling,10 phosphorochloridate,11 oxidation of phosphites12 and Atherton–Todd13 methods. In aqueous solution, polyphosphates, for example ATP and ADP, fully exist as anions and nucleophilic attack of amino acid methyl esters is prevented by electrostatic repulsion. Thus under neutral or basic conditions, no reaction with amino acid methyl esters to form phosphoramidates of adenosine in the presence of Mg2+ is observed. We thus attempted to set up a mimetic model, i.e. ADP was silylanized into its trimethylsilyl derivative which was well soluble in organic solvents such as pyridine, and the negative charges of ADP were neutralized (instead of Mg2+ in biological system) which overcame electrostatic repulsion with nucleophiles.
Under nitrogen atmosphere at room temperature 10 equiv. of trimethylsilyl chloride were added dropwise to a mixture of ADP (adenosine 5′-diphosphate disodium salt) and 2 equiv. of amino acid methyl ester hydrochloride in pyridine and stirred for two days. The addition of excess trimthylsilylchloride aimed at removing the trace amount of water in solution. The solvent was removed by reduced pressure, the residue was hydrolyzed in 2 M NH3 (aq), and extracted with diethyl ether four times, and the remaining solution was evaporated to dryness. Adenosine 5′-phosphoramidates were obtained as white solids after isolation by silica gel column chromatography using isopropyl alcohol–H2O–NH3 (aq) (13∶2∶1). Their structures were checked by 31P, 1H, 13C NMR and ESI-MS/MS.† It is interesting that nucleophilic attack of amino acid methyl esters only occurred on the α-phosphorus of ADP.
In order to illustrate the reaction mechanism of ADP with amino acid methyl esters mediated by trimthylsilylchloride, 31P NMR spectra of ADP and reaction solutions were determined as shown in Fig. 1.
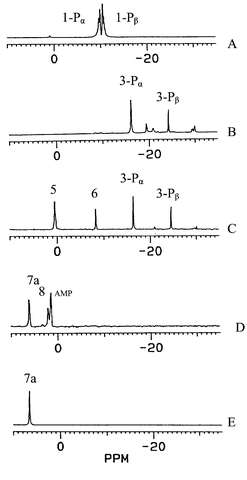 | ||
| Fig. 1 31P NMR spectra of ADP and reaction solutions. (A) ADP in water; (B) the reaction solution of ADP with trimethylsilyl chloride; (C) the reaction solution of ADP with phenylalanine methyl ester hydrochloride in the presence of trimethylsilyl chloride; (D) aqueous solution of reaction products after hydrolysis in 2 M NH3 (aq); (E) isolated product 7a. | ||
Fig. 1A shows two pairs of 31P NMR peaks at −9.56 and −9.82 ppm for ADP in water. After trimethylsilyl chloride was added to ADP in dry pyridine, and reacted for two days, 31P NMR of reaction solution exhibited two new peaks at −16.4 and −24.5 ppm corresponding to α- and β-phosphorus in compound 3. Fig. 1C shows the 31P NMR spectrum of the reaction solution of ADP with phenylalanine methyl ester in the presence of trimethylsilyl chloride. Compared with Fig. 1B, this showed the appearance of two new peaks at 0.42 and −8.27 ppm corresponding to 5a and 6. The resulting solution was evaporated to dryness, the residue was hydrolyzed in 2 M NH3, and three 31P NMR peaks at 6.44, 2.01 and 1.46 ppm corresponding to 7a, 8 and AMP, respectively, were observed. The 31P NMR chemical shift of the isolated target product 7a is at 6.44 ppm as shown in Fig. 1E. A reaction mechanism of ADP with amino acid methyl ester hydrochlorides mediated by trimethylsilyl chloride is proposed in Scheme 1. First, ADP is silylanized into 3 by trimethylsilyl chloride, then nucleophilic attack of the amino group in 4 to the α-phosphorus of 3 led to 5a and 6 which are hydrolyzed in 2 M NH3 to form 7a and 8. Hydrolysis of the remaining 3 led to anions of phosphoric acid and AMP. It is of note that reaction of 3 with 4 occurred at the α rather than the β-phosphorus which can be explained according to charge specificity and steric exclusion on the phosphorus. 31P NMR spectroscopy provided powerful evidence of the different nuture of the α and β phosphorus, the chemical shift of the β-P (δβ-P −24.62 ppm) is less than that of the α-P (δα-P −16.2 ppm), i.e. the β-phosphorus has a higher charge density than the α-phosphorus. Steric exclusion to nucleophiles is a further factor with two O-trimethylsilyl groups on the β-phosphorus shielding it from nucleophilic attack.
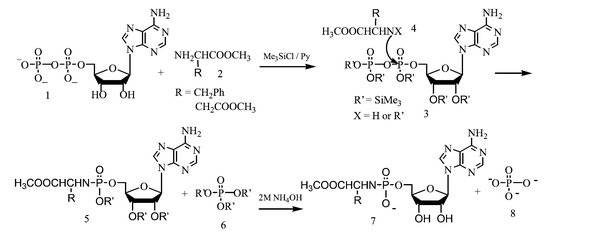 | ||
| Scheme 1 Reaction mechanism of ADP with amino acid methyl ester hydrochlorides mediated by trimethylsilyl chloride. | ||
Several new signals were observed in Fig. 1B and C. First, three small peaks at −19.58, −20.94 and −29.90 ppm can be attributed to pentacoordinated phosphorane intermediates formed by 3-Pα and 3-Pβ with pyridine as shown in Scheme 2 and as suggested by Mitin and Gliskaya14 and Yamazaki and Higashi.15 Secondly, the signals at 0.42 ppm corresponding to 5a and at −16.2 ppm corresponding to 3-Pα, are single peaks, and the possible trimethylsilylphosphate intermedates are shown in Scheme 3. Similar pentacoordinated silicons have been reviewed by Holmes.16 P–O–P coupling was not observed: P–O–P coupling constants could be be small because of the presence of the trimethylsilyl group.
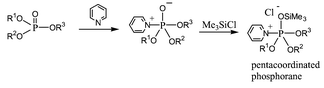 | ||
| Scheme 2 | ||
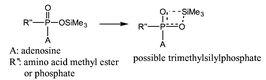 | ||
| Scheme 3 | ||
In conclusion, reaction of ADP with amino acid methyl esters mediated by trimethylsilyl chloride in pyridine produced adenosine 5′-phosphoramidates in good yields, which could be good mimetic model for the reaction of polyphosphates with nucleophiles in biological processes. This new and efficient one-pot method can be generally applied to the synthesis of other nucleoside 5′-phosphoramidate prodrugs.
The work was supported by the National Natural Science Foundation of China (No.29902003), the National Science and Technology Committee of China, the Chinese Education Ministry and Tsinghua University.
Notes and references
- M. J. Roth and J. Hurwitz, J. Biol. Chem., 1984, 259, 13488 CAS.
- R. Toyama, K. Mizumoto, Y. Nakahara, T. Tatsuno and Y. Kaziro, EMBO J., 1983, 12, 2195.
- S. Shuman and B. Schwer, Mol. Microbiol., 1995, 17, 405 CAS.
- S. Shuman, M. I. Surks, H. M. Furneaux and J. Hurwitz, J. Biol. Chem., 1980, 255, 11588 CAS.
- S. Shuman and J. Hurwitz, Proc. Natl. Acad. Sci. USA, 1981, 78, 187 CAS.
- K. Hakansson and D. B. Wigley, Proc. Natl. Acad. Sci. USA, 1998, 95, 1505 CrossRef CAS.
- C. McGuigan, D. Cahard, H. M. Sheeka, E. DeClercq and J. Balzarini, J. Med. Chem., 1996, 39, 1748 CrossRef CAS.
- C. R. Wagner, E. J. McIntee, R. F. Schinazi and T. W. Abraham, Bioorg. Med. Chem. Lett., 1995, 5, 1819 CrossRef CAS.
- J. Balzarini, H. Egberink, K. Hartman, D. Cahard, T. Vahlenkamp, H. Thormar, E. DeClercq and C. McGuigan, Proc. Mol. Pharmacol., 1996, 50, 1207 Search PubMed.
- T. W. Abraham, T. I. Kalman, E. J. McIntee and C. R. Wagner, J. Med. Chem., 1996, 39, 4569 CrossRef CAS.
- C. McGuigan, K. G. Devine, T. J. O’Connor and D. Kinchington, Antiviral Res., 1991, 15, 255 CrossRef CAS.
- T. W. Abraham and C. R. Wagner, Nucleosides Nucleotides, 1994, 13, 1891 Search PubMed.
- F. R. Atherton, H. T. Openshaw and A. R. Todd, J. Chem. Soc., 1945, 660 RSC; F. R. Atherton and A. R. Todd, J. Chem. Soc., 1947, 674 RSC.
- Y. V. Mitin and O. V. Glinskaya, Tetrahedron Lett., 1969, 5267 CrossRef CAS.
- N. Yamazaki and F. Higashi, Tetrahedron Lett., 1972, 5047 CrossRef CAS.
- R. R. Holmes, Chem. Rev., 1996, 96, 927 CrossRef CAS.
Footnote |
† Spectral data: compound 7a: yield: 52%. 31P NMR (H2O): δ 6.82; 1H NMR (D2O): δ 2.84 (d, 2H, β-CH2, 3JHH 7.00 Hz), 3.67 (s, 3H, OCH3), 3.91 (m, 3H, α-CH, 5′-CH2), 4.34 (m, 1H, 4′-CH), 4.49 (t, 1H, 3′-CH), 4.77 (1H, 2′-CH), 6.10 (d, 1H, 1′-CH, 3JHH 5.50 Hz), 7.00–7.16 (m, 5H, Ph), 8.20 (s, 1H, 8-CH), 8.41 (s, 1H, 2-CH); 13C NMR (D2O): δ 42.61 (d, β-CH2, 3JPC 6.38 Hz), 55.07 (OCH3), 58.99 (α-CH), 66.39 (d, 5′-CH2, 2JPC 3.00 Hz), 73.06 (2′-CH), 76.71 (3′-CH), 86.44 (d, 4′-CH, 3JPC 8.88 Hz), 89.76 (1′-CH), 121.24 (5-C), 129.34, 130.95, 131.63, 139.30 (Ph), 142.35 (8-CH), 151.58 (4-C), 155.46 (2-CH), 158.08 (6-C), 179.16 (CO). Negative ion ESI-MS: m/z 507 [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H]−; Compound 7b: yield: 46%. 31P NMR (H2O): δ 6.35; 1H NMR (D2O): δ 2.61 (m, 2H, β-CH2), 3.46 (s, 3H, OCH3), 3.60 (s, 3H, OCH3), 3.88 (m, 1H, α-CH), 3.92 (m, 2H, CH, 5′-CH2), 4.27 (m, 1H, 4′-CH), 4.42 (t, 1H, 3′-CH), 4.74 (1H, 2′-CH), 6.02 (d, 1H, 1′-CH, 3JHH 6.00 Hz), 8.17 (s, 1H, 8-CH), 8.39 (s, 1H, 2-CH); 13C NMR (D2O): δ 40.88 (β-CH2, 3JPC 4.12 Hz), 53.80 (OCH3), 54.78 (OCH3), 55.42 (d, α-CH), 66.32 (d, 5′-CH2, 2JPC 4.25 Hz), 73.06 (2′-CH), 76.53 (3′-CH), 86.60 (d, 4′-CH, 3JPC 9.12 Hz), 89.51 (1′-CH), 121.32 (5-C), 142.36 (8-CH), 151.70 (4-C), 155.49 (2-CH), 158.21 (6-C), 175.81 (CO), 177.68 (CO, 3JPC 6.50 Hz). Negative ion ESI-MS: m/z 489 [M H]−; Compound 7b: yield: 46%. 31P NMR (H2O): δ 6.35; 1H NMR (D2O): δ 2.61 (m, 2H, β-CH2), 3.46 (s, 3H, OCH3), 3.60 (s, 3H, OCH3), 3.88 (m, 1H, α-CH), 3.92 (m, 2H, CH, 5′-CH2), 4.27 (m, 1H, 4′-CH), 4.42 (t, 1H, 3′-CH), 4.74 (1H, 2′-CH), 6.02 (d, 1H, 1′-CH, 3JHH 6.00 Hz), 8.17 (s, 1H, 8-CH), 8.39 (s, 1H, 2-CH); 13C NMR (D2O): δ 40.88 (β-CH2, 3JPC 4.12 Hz), 53.80 (OCH3), 54.78 (OCH3), 55.42 (d, α-CH), 66.32 (d, 5′-CH2, 2JPC 4.25 Hz), 73.06 (2′-CH), 76.53 (3′-CH), 86.60 (d, 4′-CH, 3JPC 9.12 Hz), 89.51 (1′-CH), 121.32 (5-C), 142.36 (8-CH), 151.70 (4-C), 155.49 (2-CH), 158.21 (6-C), 175.81 (CO), 177.68 (CO, 3JPC 6.50 Hz). Negative ion ESI-MS: m/z 489 [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H]−. H]−. |
| This journal is © The Royal Society of Chemistry 2003 |