Chiral quantification of D-, L-, and meso-tartaric acid mixtures using a mass spectrometric kinetic method
Lianming
Wu
,
Rebecca L.
Clark
and
R. Graham
Cooks
*
Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA. E-mail: cooks@purdue.edu; Fax: 1-765-494-9421; Tel: 1-765-494-5265
First published on 3rd December 2002
Abstract
Accurate quantification of the optical isomers in a ternary mixture of D-, L-, and meso-tartaric acids is achieved using electrospray ionization tandem mass spectrometry for in-situ metal complex formation and a three-point calibration method to quantify the dissociation kinetics.
In a previous communication1 we showed that fragmentation rates of metal complexes in the mass spectrometer, treated by a kinetic method, permit rapid enantiomeric determination of α-hydroxy acids. Now we report the first use of mass spectrometry for optical isomer quantification of a ternary mixture. This novel method should be applicable to ternary and higher mixtures of optical isomers. As such, it may provide a solution to the challenging problem of how to perform chiral quantification of multiple chiral-center drugs and their metabolites rapidly and accurately, a requirement imposed by national pharmaceutical regulatory bodies and a topic of great interest in pharmacology.2 It may be also useful in developing chiral morphing techniques,3 which allow utilization of the spatial diversity of multiple chiral centers to produce drug candidates with improved efficiency, stability, membrane permeability, and oral availability, as well as decreased toxicity and side effects.
Several mass spectrometric methods for chiral analysis have already been developed, including guest–host adducts formation,4 ion/molecule reactions,5,6 collision-induced dissociation of diastereomeric adducts,7 and solution-phase kinetic resolution followed by mass spectrometry.8 However, none of them aims at chiral ternary mixture analysis. The kinetic method9 as used for chiral analysis distinguishes optical isomers via their transition metal (MII)-bound trimeric cluster ions by investigating the dissociation kinetics.10,11 In general, singly-charged cluster ions [MII(ref*)2(A) − H]+ are mass-selected and dissociated in an ion trap mass spectrometer to form the dimeric cluster ions [MII(ref*)(A) − H]+ and [MII(ref*)2 − H]+ by competitive loss of the neutral reference compound ref* and the analyte A, respectively. The difference in stability of the diastereomeric ions [MII(ref*)(A) − H]+ due to the two configurations of the analyte A, results in a relative abundance ratio (R, eqn. 1) that depends on the isomeric composition of the analyte A:
| R = [MII(ref*)(A) − H]+/[MII(ref*)2 − H]+ | (1) |
| Δ(ΔG) = αD*Δ(ΔG)D + αL*Δ(ΔG)L + (1 − αD − αL)*Δ(ΔG)meso | (2) |
| ln R = αD*lnRD + αL*lnRL + (1 − αD − αL)*lnRmeso | (3) |
If the same analyte is also measured under a second set of conditions, a second set of equations is obtained and the system of two unknowns can be solved. Note that small changes in conditions are less useful than changes to both the nature of the reference and the choice of metal ion. The latter (more ‘orthogonal’ systems) gives relatively larger distinctions for all three optical isomers. In previous work on α-hydroxy acids,14 we identified two candidate orthogonal systems: (i) CoII as the central metal ion and L-DOPA as the reference ligand; (ii) Ni II as the central metal ion and N-acetyl-L-Phe as the reference ligand. These systems were therefore selected for the analysis of the ternary tartaric acid mixtures and typical mass spectra showing the differentiation of D-, L-, and meso-tartaric acid using system (ii) are illustrated in Fig. 1. Data for system (i) is not shown but the measured branching ratios for both systems (i) and (ii) were obtained. To illustrate the additional contribution from meso-tartaric acid, three-point calibration curves constructed using the measured branching ratios are displayed versus the molar fraction of D-tartaric acid (αD) (Fig. 2). Using Cartesian coordinates, the measured R values can be converted into two groups of points: D1(100, −1.61), L1(0, −2.27), meso1(0, −1.90) for system (i) shown as open-diamond symbols and D2(100, 0.0770), L2(0, −0.728), meso2(0, −0.442) for system (ii), shown as filled-triangle symbols. Note that the points D, L, and meso correspond to cases in which the analyte is pure D, pure L, and pure meso, respectively. For the pure enantiomeric D/L-tartaric acid pair, the two-point calibration curves for the two experimental conditions mentioned above are represented by Curve(D1-L1) and Curve(D2-L2), respectively. With the additional contributions of meso-tartaric acid, these two curves shift upwards, by an amount that depends on the mole fraction of meso-compound in the sample. For all possible compositions, the logarithmic value of each measured ratio, ln R, will fall into the area of the triangle(D1–L1–meso1, dotted line) or triangle(D2–L2–meso2, solid line). This three-point calibration relationship is described using eqn. 3, which takes the forms shown in eqns. 4 and 5 when substituting the actual measured values of RD, RL, and Rmeso for these two systems.
| ln R(i) = (−1.61)*αD + (−2.27)*αL + (−1.90)*(1 − αD − αL) | (4) |
| ln R(ii) = (0.08)*αD + (0.728)*αL + (0.442)*(1 − αD − αL) | (5) |
![MS/MS product ion spectra of [NiII(N-Acetyl-L-Phe)2(A) − H]+: A = (a) d-tartaric acid; (b) meso-tartaric acid; and (c) L-tartaric acid using helium CID at an activation level of 275 mV in an ion trap.](/image/article/2003/CC/b210294c/b210294c-f1.gif) | ||
| Fig. 1 MS/MS product ion spectra of [NiII(N-Acetyl-L-Phe)2(A) − H]+: A = (a) D-tartaric acid; (b) meso-tartaric acid; and (c) L-tartaric acid using helium CID at an activation level of 275 mV in an ion trap. | ||
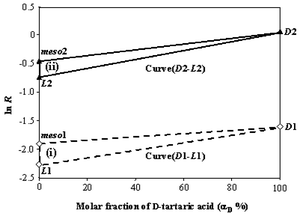 | ||
| Fig. 2 Three-point calibration diagram for chiral quantification of a ternary mixture of tartaric acid using two separate systems: (i) CoII as the central metal ion and L-DOPA as the reference ligand; (ii) NiII as the central metal ion and N-Acetyl-L-Phe as the reference ligand. Values corresponding to each point are averages based on triplicate measurements made on separate occasions with less than ca. 2% error. | ||
Chiral quantification of ternary mixtures of tartaric acid was performed using samples with several representative compositions, the results being summarized in Table 1. The experimentally measured molar fractions are consistent with the actual compositions and they show that less than 1% mole fraction for each form of tartaric acid can be determined with relative errors that range from 0.5% to 6.0%. The average relative errors for D-tartaric acid, L-tartaric acid, and meso-tartaric acid are 1.8%, 2.4%, 2.8%, respectively. The present results show that the kinetic method allows quantitative chiral analysis of a ternary mixture of multiple chiral-center molecules.
| Measured R | Actual | Experimental | Relative error(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Co/L-DOPA | Ni/N-Acetyl- L-Phe | %D | %L | %meso | %D | %L | %meso | D | L | meso |
| a CID activation time and energy is optimized and kept constant when the same transition metal and reference ligand are used. | |||||||||||
| 1 | 0.177 | 0.851 | 65 | 30 | 5 | 65.3 | 29.5 | 5.2 | 0.5 | 1.7 | 4.0 |
| 2 | 0.191 | 1.01 | 90 | 5 | 5 | 89.5 | 5.3 | 5.2 | 0.6 | 6.0 | 4.0 |
| 3 | 0.143 | 0.639 | 10 | 20 | 70 | 9.6 | 19.7 | 70.7 | 4.0 | 1.5 | 1.0 |
| 4 | 0.109 | 0.509 | 5 | 90 | 5 | 4.9 | 90.3 | 4.9 | 2.0 | 0.3 | 2.0 |
| Average: | 1.8 | 2.4 | 2.8 | ||||||||
When choosing two suitable systems (two different sets of the central metal ions and the reference ligands), the kinetic method offers sensitivity and accuracy for chiral quantification of a ternary mixture similar to that reported for binary mixtures.1,14 It is important to note that the use of pure calibration samples of the three isomers is not needed: one simply requires that three samples of known isomeric composition be available. In addition, the quotient ratio method15 in which multiple experiments are done using enantiomeric reference ligands could be advantageous. This procedure reduces the requirement for analytes of known composition from three to two. Extension of the procedures described in this paper to mixtures of four optical isomers using a four-point calibration kinetic method should be straight forward.
This work was supported by the U.S. DOE, Office of Basic Energy Sciences and the National Science Foundation (CHE 97-32670). L.W. thanks fellowship support from Merck & Co.
Notes and references
- W. A. Tao, L. Wu and R. G. Cooks, Chem. Commun., 2000, 20, 2023 RSC.
- S. C. Stinson, Chem. Eng. News, 2000, 78, 55 Search PubMed.
- J. C. Fromme and G. L. Verdine, Nature Struct. Biol., 2002, 9, 544 Search PubMed.
- M. Shizuma, H. Imamura, Y. Takai, H. Yamada, T. Takeda, S. Takahashi and M. Sawada, Int. J. Mass Spectrom., 2001, 210–211, 585 CrossRef.
- Y. J. Liang, J. S. Bradshaw, R. M. Izatt, R. M. Pope and D. V. Dearden, Int. J. Mass Spectrom., 1999, 187, 977 CrossRef.
- J. Ramirez, F. He and C. B. Lebrilla, J. Am. Chem. Soc., 1998, 120, 7387 CrossRef CAS.
- T. T. Dang, S. F. Pedersen and J. A. Leary, J. Am. Soc. Mass Spectrom., 1994, 5, 452 CrossRef CAS.
- J. Guo, J. Wu, G. Siuzdak and M. G. Finn, Angew. Chem., Int. Ed., 1999, 38, 1755 CrossRef CAS.
- R. G. Cooks and P. S. H. Wong, Acc. Chem. Res., 1998, 31, 379 CrossRef CAS.
- A. Filippi, A. Giardini, S. Piccirillo and M. Speranza, Int. J. Mass Spectrom., 2000, 198, 137 CrossRef CAS.
- M. G. Finn, Chirality, 2002, 14, 534 CrossRef CAS.
- L. C. R. Pasteur, Hebd. Seance Acad. Sci. Paris, 1848, 26, 535 Search PubMed.
- M. O. Lorenzo, C. J. Baddeley, C. Muryn and R. Raval, Nature, 2000, 404, 376 CrossRef CAS.
- 14 L. Wu, W. A. Tao and R. G. Cooks, Anal. Bioanal. Chem., 2002, 373, 618 Search PubMed.
- 15 W. A. Tao, R. L. Clark and R. G. Cooks, Anal. Chem., 2002, 74, 3783 CrossRef.
| This journal is © The Royal Society of Chemistry 2003 |
