Formation and observation of dimers of a metal complex with long alkyl side chains aligned on a graphite surface
Ichiro
Sakata
and
Kazuo
Miyamura
*
Department of Chemistry, Faculty of Science, Science University of Tokyo, 1-3 Kagurazaka, Shinjuku, Tokyo, 162-8601, Japan
First published on 5th December 2002
Abstract
Scanning tunnelling microscope was successfully applied to observe self-assembled molecular images of (bis(5-dodecylsalicylidene)ethylenediaminato)nickel(II) in the form of dimers on highly oriented pyrolytic graphite
Compounds having long alkyl side chains are known to attain the ability to aggregate at interfaces and to decrease surface tension. These compounds are denoted surfactants and exhibit a variety of properties, such as surface activation, monolayer formation, detergency, etc. All these characteristic properties of surfactants are a consequence of molecular aggregation. Understanding the structure of the aggregates is important to recognise the properties of the surfactants induced by aggregation. Such considerations also hold for metal complexes containing long alkyl chains.
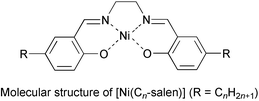
In previous papers, we have reported the aggregating properties of nickel(II) complexes of salen1 substituted with two long alkyl (CnH2n+1) groups, (bis(5-alkylsalicylidene)ethylenediaminato)nickel(II) [Ni(Cn-salen)].2 A single-crystal X-ray analysis performed on the butyl substituted analogue [Ni(C4-salen)] suggested the formation of dimers, the structure of which was concluded to be formed by π–π stacking of the salen moieties, and also by CH–π interactions between the salen moieties and the alkyl chains.3 This structure is thus characteristic for complexes substituted with long alkyl chains.
We have also reported on the formation of monolayers of [Ni(Cn-salen)] at the air–water interface by the Langmuir–Brodgett (LB) method,4 and on the observation of the aggregating structure of [Ni(C4-salen)] molecules in the monolayers by scanning tunnelling microscopy (STM). The monolayer scooped onto highly oriented pyrolytic graphite (HOPG) was found to be constituted of dimers of [Ni(C4-salen)]. The dimeric structure therein determined from the STM image was similar to that observed in the crystalline state. This structure, however, is a consequence of the pressure applied to the monolayer. In the preparation procedure of the sample by the LB method the monolayer is compressed and the molecules are jammed up together to form forced aggregates. It is not yet certain whether [Ni(Cn-salen)] molecules self-assemble to form self-aggregates of characteristic structure. In this paper the molecular images of the self-assembled dodecyl substituted analogue [Ni(C12-salen)] are reported. Although many molecular images of liquid crystals,5 metallophthallocyanines,6 organic solvents,7etc. have been reported, this is the first observation at molecular resolution of self-assembled surface-active metal-complex forming dimers.
[Ni(C12-salen)] was prepared by the procedure described in the literature8 and the chemical structure was confirmed by 1H NMR spectroscopy. In the present experiment a 1.0 mM solution of [Ni(C12-salen)] in dichlorobenzene was dropped onto a freshly cleaved surface of HOPG and used immediately in the measurement. STM measurements were performed with Digital Instruments Nanoscope II/E STM equipment under ambient conditions. STM tips were prepared by electrochemical etching of Pt/Ir(80∶20) wire according to the reported procedures.6 All STM images were obtained with a constant current mode and the tunnel current and sample bias voltage were set to 400 pA and −900 mV, respectively.
An ordered image due to adsorption of [Ni(C12-salen)] on HOPG gradually appeared from about 30 min after the preparation of the sample. Fig. 1(a) shows a 15 nm × 15 nm STM image of a [Ni(C12-salen)] adsorbed HOPG surface thus obtained. Bright flower-like structures are aligned regularly over the whole area. The periodicities of the bright images are 1.7 and 2.2 nm in the directions shown in the figure. Since the C–C bond length of carbons at the HOPG surface is 0.142 nm, the observed periodicities are an order of manitude higher. The size of the dichlorobenzene molecules used as a solvent are also too small to cause the observed pattern. The acute angle of the unit cell shown as a rhomboid in Fig. 1 is 80°. The area of the unit cell is thus calculated as 3.7 nm2, which corresponds to the area of 70 hexagonal units of carbon atoms on the HOPG surface. Accordingly, the image is not that of HOPG nor of dichlorobenzene, but should be of [Ni(C12-salen)].
![15 nm × 15 nm STM image of [Ni(C12-salen)] on HOPG.](/image/article/2003/CC/b208893b/b208893b-f1.gif) | ||
| Fig. 1 15 nm × 15 nm STM image of [Ni(C12-salen)] on HOPG. | ||
Fig. 2(a) shows an increased magnification image which clearly shows that the flower-like image is a stack of two molecules of [Ni(C12-salen)] forming a dimer as illustrated in Fig. 2(b). The length and width of the left-side half of the flower-like image are about 1.4 nm and 0.37 nm, respectively. This longitudinal value is a similar value to the length of the salen moiety obtained by X-ray crystallographic analysis. However, the fact that the width is shorter than the width of salen moiety (0.69 nm), indicates that [Ni(C12-salen)] molecules are inclined by about 58° from the HOPG surface. Hence the left-side molecule is overlapping the right-side molecule in the image. This structure of the dimer differs from that observed in the STM image of the forced-dimer prepared by the LB method, but resembles that in chloroform solution estimated by NMR analysis.2 The expected length of the dodecyl group is about 1.0 nm, which can be distinguished as a grey image indicated by an arrow. The dodecyl groups show no apparent interaction with the complex molecules, but seem to lie over the HOPG surface.
![(a) 6 nm × 6 nm STM image of [Ni(C12-salen)] and (b) proposed structure of the dimer in the inlet of (a). Applied bias voltage: −900 mV, current: 300 pA.](/image/article/2003/CC/b208893b/b208893b-f2.gif) | ||
| Fig. 2 (a) 6 nm × 6 nm STM image of [Ni(C12-salen)] and (b) proposed structure of the dimer in the inlet of (a). Applied bias voltage: −900 mV, current: 300 pA. | ||
In the STM imaging of weakly adsorbed molecules, which is often the case for samples prepared by the self-assembly procedure, the movement of the adsorbed molecules induced by the tip often disturbs the measurement. However, the introduction of dodecyl chains to the complex molecule effectively anchored the molecule on the HOPG surface. This explains why clear molecular images could be obtained despite the self-assembly procedure. Further the forced-assembly procedure (LB method) on [Ni(C12-salen)] is reported to result in the formation of a monolayer similar to that of [Ni(C4-salen)].4 The present study thus also showed the difference in the structure of aggregates prepared by forced- and self-assembly procedures.
Notes and references
- Salen is an abbreviation of bis(salicylidene)ethylenediaminato.
- K. Miyamura, K. Sato and Y. Gohshi, Bull. Chem. Soc. Jpn., 1989, 62, 45 CAS.
- K. Miyamura, A. Mihara, T. Fujii, Y. Gohshi and Y. Ishii, J. Am. Chem. Soc., 1995, 117, 2377 CrossRef CAS.
- K. Miyamura and T. Fujii, Bull. Chem. Soc. Jpn., 2000, 73, 365 CrossRef.
- J. K. Spong, H. A. Mizes, L. J. LaComb, Jr., M. M. Dovek, J. E. Frommer and J. S. Foster, Nature (London), 1989, 338, 137 CrossRef CAS; D. P. E. Smith, H. Herber, Ch. Gerber and G. Binnig, Science, 1989, 245, 43 CAS.
- R. H. Lippel, R. J. Wilson, M. D. Miller, Ch. Woll and S. Chiang, Phys. Rev. Lett., 1989, 62, 171 CrossRef CAS.
- H. Ohtani, R. J. Wilson, S. Chiang and C. M. Mate, Phys. Rev. Lett., 1988, 60, 2398 CrossRef.
- L. Libioulle, Y. Houbion and J.-M. Gilles, Rev. Sci. Instrum., 1995, 66, 97 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2003 |