Light induced excited high spin-state trapping in [FeL2](BF4)2 (L = 2,6-di(pyrazol-1-yl)pyridine)
Victoria A.
Money
a,
Ivana
Radosavljevic Evans
a,
Malcolm A.
Halcrow
b,
Andrés E.
Goeta
a and
Judith A. K.
Howard
*a
aChemistry Department, Durham University, South Road, Durham, UK DH1 3LE. E-mail: j.a.k.howard@durham.ac.uk
bDepartment of Chemistry, University of Leeds, Leeds, UK LS2 9IT
First published on 5th December 2002
Abstract
The spin-crossover complex [FeL2](BF4)2 undergoes a LIESST transition at 30 K on irradiation; the structures of the low-spin ground and high-spin metastable states at this temperature are presented.
The first iron(II) spin-crossover compound was reported by Baker and Bobonich in 1964.1 Since then there has been much interest in these materials due to their potential for applications such as information storage, molecular switches and visual displays.2 In the mid 1980s Decurtins et al. reported the first case of a transition from low to high spin caused by irradiation, the LIESST effect (Light Induced Excited High Spin-State Trapping).3 This opened up the possibility of these materials being used in optical devices. The metastable high-spin states, HS-2, formed in this way have been found to have very long lifetimes at low temperatures with the highest reported LIESST temperature, as defined by Létard et al.,4 being 130 K.5 Crystallographic structures of the HS-2 state have been reported in only a small number of cases, two recent examples being the studies by Kusz et al. in 20016 and Marchivie et al. in 2002.7
 | ||
| Fig. 1 L = 2,6-Di(pyrazol-1-yl)pyridine. | ||
In comparison with many spin-crossover compounds in which the transition is very gradual and the low-spin state, LS, is only stable at very low temperatures, [FeL2](BF4)2 (Fig. 1)† shows an abrupt thermal spin transition centred at 260 K with a small hysteresis loop of 3 K.8 The relatively high temperature of the transition combined with the molecular bistability indicated by the hysteresis makes this compound very interesting for the applications mentioned above. We have therefore undertaken a further X-ray diffraction study of this system in order to probe the changes in its molecular and crystal structure over a wide temperature range.
Single crystal X-ray diffraction data† were collected at 290(2), 120(2), 30(2) K and after irradiation at 30(2) K. The structures of the high-spin state at 290(2) K, HS-1, and 120(2) K, LS, show good agreement with the published data.8 On flash freezing to 30(2) K the crystal remains in the monoclinic space group P21. Unlike the isomeric spin crossover compound [Fe(bpp)2](BF4)2 (bpp = 2,6-bis(pyrazol-3-yl)pyridine),9 [FeL2](BF4)2 is not trapped in a metastable state on flash freezing, most likely owing to the relatively high temperature of the thermal spin transition. Flash freezing of the sample was necessary due to degradation of the crystals on slow cooling, which it was thought might be due to a further phase change. However, the variation of the unit cell volume between 30 K and 260 K, accurately determined from variable temperature powder X-ray diffraction data (Fig. 2), shows an abrupt increase which corresponds to the transition to the HS-1 and eliminates the possibility of an additional phase change in this temperature range. From these data, a thermal expansion tensor was calculated and the obtained volume coefficient of thermal expansion at 50 K is αV = 1.07 × 10−4 K−1. The spin transition is accompanied by a thermochromic effect in which the crystal changes colour from yellow in the high-spin state to brown in the low-spin state. Table 1 shows several key parameters which differ for each spin state.
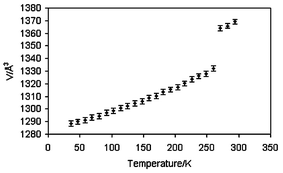 | ||
| Fig. 2 Change in unit cell volume on warming from 30(2) to 300(2) K determined by variable temperature powder X-ray diffraction. | ||
| Spin state | T/K | V/Å3 | β/° | Mean Fe–N/Å | Bite angle | BF4− |
|---|---|---|---|---|---|---|
| HS-1 | 290 | 1373.3(5) | 95.67(3) | 2.166(6) | 73.4(2) | Disordered |
| LS | 120 | 1308.6(5) | 98.37(3) | 1.953(2) | 80.09(8) | Ordered |
| LS | 30 | 1288.8(1) | 98.575(1) | 1.950(2) | 80.08(8) | Ordered |
| HS-2 | 30 | 1318.1(5) | 97.15(1) | 2.165(2) | 73.52(7) | Ordered |
![Molecular structure of low-spin [FeL2]2+ at 30 K showing the numbering scheme used for the complex cation. Hydrogen atoms have been omitted for clarity. Thermal ellipsoids are at 90% probability.](/image/article/2003/CC/b210146g/b210146g-f3.gif) | ||
| Fig. 3 Molecular structure of low-spin [FeL2]2+ at 30 K showing the numbering scheme used for the complex cation. Hydrogen atoms have been omitted for clarity. Thermal ellipsoids are at 90% probability. | ||
The crystal was irradiated with a He–Ne laser (λ = 632.8 nm, 15 mW) at 30(2) K for 30 min. It was noted that after irradiation the crystal had changed colour from brown to yellow implying that the HS-2 state had been formed. Data were collected on the irradiated crystal at 30(2) K. The Fe–N bonds have an average length of 2.165(2) Å, indicative of Fe(II) in the HS state. This increase in average ligand–metal bond length of 0.213 Å is comparable to reported results for spin transitions of Fe(II) bonded to six nitrogen donors.10,11 The volume of the unit cell was found to be 1318.1(5) Å3. This is an increase of 29.3 Å3 equal to 2.3% on going from the LS to the HS-2 state. This is in good agreement with the 2.6% increase calculated for the thermal spin transition8 and in the range expected for mononuclear Fe(II) materials.12,13 However the similarity between the increase in volume, ΔVSC, in the thermal and light induced transitions is unusual. In the two cases mentioned above ΔVSC has been found to be significantly less for the light induced transition than reported for the thermal transition.6,7 Crystalline [FeL2](BF4)2 is very tightly packed with a non hydrogen atom volume of 15 Å3 and this may account for the similarity in the ΔVSC values. Significant differences in the molecular geometry of the two spin states are not restricted to the ligand metal bonds. There is a shortening of all N–N bonds on going from the LS to the HS-2 state which is most pronounced in those rings forming the shortest Fe–N bonds. This decrease in bond length is probably due to an increase in t2g back bonding in the HS-2 state; it is thought that the shorter metal ligand bonds in the LS state increase the degree of backbonding due to enhanced overlap between the orbitals. As a result of the change in Fe–N bond lengths the ligand bite angle is decreased from an average value of 80.08(8)° for the LS complex to 73.52(7)° for the HS-2 state. The N3–Fe1–N8 bond angle changes from 177.7(1)° for the LS state to 169.68(8)° for the HS-2 state. All the Fe–N bonds in the HS-2 structure at 30(2) K are within an esd of the equivalent bond lengths at room temperature. The structure of the HS-2 cation at 30(2) K is very similar to that determined at room temperature, however no disorder is found in the BF4− anions at 30(2) K.
Variation of the unit cell volume with temperature after irradiation shows an abrupt decrease from 1333(2) to 1302(7) Å, Fig. 4. The discontinuity coincides with the colour change of the crystal from yellow to brown. The LIESST transition, as determined by X-ray diffraction measurements, therefore takes place between 80(2) K and 85(2) K. The volume coefficient of thermal expansion of HS-2 at 50 K is αV = 0.71 × 10−4 K−1. The changes in cell parameters during relaxation from HS-2 to LS show a pronounced anisotropy. The same trend is seen in the thermal transition between LS and HS-1. The change of the crystallographic c parameter is more than five times that in either a or b. This could be due to the Fe–N bonds to the central pyridine ring (Fe–N3 and Fe–N8), which lie in the direction of the c axis and show the largest change in length over the transition. It is possible that intermolecular bonding also plays a part: edge-to-face and face-to-face interactions lie along the a and b axis whilst there is some hydrogen bonding between CH and F atoms along the c axis.
2 on warming after irradiation.](/image/article/2003/CC/b210146g/b210146g-f4.gif) | ||
| Fig. 4 Change in unit cell volume of a single crystal of [FeL2](BF4)2 on warming after irradiation. | ||
Photomagnetic measurements are underway to confirm the exact temperature of the transition, the degree of conversion obtained by irradiation and to determine the effect of continued irradiation on the LIESST temperature. Planned infrared spectroscopy on the irradiated sample will enable us to determine the rate of relaxation from the HS-2 state to the ground LS state.
The authors would like to thank Dr A. Beeby for assistance with the LIESST experiments, Dr J. S. O. Evans for help with the powder diffraction experiments and the EPSRC for a studentship (V. A. M.) and for a Senior Research Fellowship (J. A. K. H.).
Notes and references
- W. A. Baker and H. M. Bobonich, Inorg. Chem., 1964, 3, 1184 CrossRef CAS.
- P. Gütlich, A. Hauser and H. Spiering, Angew. Chem. Int., Ed. Engl., 1994, 33, 2024 CrossRef; P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419 RSC.
- S. Decurtins, P. Gütlich, K. M Hasselbach, H. Spiering and A. Hauser, Inorg. Chem., 1985, 24, 2174 CrossRef CAS.
- J-F. Létard, L. Capes, G. Chastanet, N. Moliner, S. Létard, J-A. Real and O. Kahn, Chem. Phys. Lett., 1999, 313 , 115–120 CrossRef CAS.
- S. Hayami, Z. Gu, Y. Einaga, Y. Kobayasi, Y. Ishikawa, Y. Yamada. A. Fujishima and O. Sato, Inorg. Chem., 2001, 40, 3240 CrossRef CAS.
- J. Kusz, H. Spiering and P. Gütlich, J. Appl. Crystallogr., 2001, 34, 229 CrossRef CAS.
- M. Marchivie, P. Guionneau, J. A. K. Howard, A. E. Goeta, G. Chastanet, J-F. Létard and D. Chasseau, J. Am. Chem. Soc., 2002, 124, 194 CrossRef CAS.
- J. M. Holland, J. A. McAllister, C. A. Kilner, M. Thornton-Pett, A. J. Bridgeman and M. A. Halcrow, J. Chem. Soc., Dalton Trans., 2002, 548 RSC; J. M. Holland, J. A. McAllister, Z. Lu, C. A. Kilner, M. Thornton-Pett and M. A. Halcrow, Chem. Commun., 2001, 577 RSC.
- S. Marcén, L. Lecren, L. Capes, H. A. Goodwin and J-F. Létard, Chem. Phys. Lett., 2002, 359, 87–95 CrossRef CAS.
- P. Guionneau, C. Brigouleix, Y. Barrans, A. E. Goeta, J-F. Létard, J. A. K. Howard, J. Gaultier and D. Chasseau, C. R. Acad. Sci., Ser. IIc: Chim., 2001, 4, 161 CrossRef CAS.
- E. König, Prog. Inorg. Chem., 1987, 35, 527 Search PubMed.
- Y. Garcia, P. Guionneau, G. Bravic, D. Chasseau, J. A. K. Howard, O. Kahn, V. Ksefontov, S. Reiman and P. Gütlich, Eur. J. Inorg. Chem., 2000, 1531 CrossRef CAS.
- P. Guionneau, M. Marchivie, G. Bravic, J-F. Létard and D. Chasseau, J. Mater. Chem., 2002, 12, 2546 RSC.
- A. E. Goeta, L. K. Thompson, C. L. Sheppard, S. S. Tandon, C. W. Lehmann, J. Cosier, C. Webster and J. A. K. Howard, Acta. Crystallogr., Sect. C, 1999, 55, 1243 CrossRef.
- Variable temperature powder diffraction studies made possible by EPSRC/JREI Grant number GR/M35222..
Footnote |
| † Synthesis: Crystals and polycrystalline powder of [FeL2](BF4)2 were synthesised as described in the literature.8Crystal data: C22H18N10FeB2F8, Mr = 651.93, monoclinic, P21, Z = 2. 30(2) K: a = 8.4098(4) Å, b = 8.4731(4) Å, c = 18.2909(9) Å, β = 98.575(1)°, V = 1288.8(1) Å3Dc = 1.680 Mg m−3, μ = 0.679 mm−1, λ(Mo-Kα) = 0.71073 Å. Selected crystal, 0.20 × 0.18 × 0.06 mm, mounted on a hair in fluoropolyether oil and flash frozen in a chilled helium gas flow at 30(2) K using an Oxford Cryosystems HELIX.14 11476 reflections (2.65 < θ < 27.45°) collected on Bruker SMART–CCD diffractometer (ω-scan, 0.3°/frame) yielding 5434 unique data (Rint = 0.0335). The structure was solved by direct methods and refined by full matrix least squares based on F2 for all data using SHELXL software. All non-hydrogen atoms were refined with anisotropic refinement factors, H-atoms were placed geometrically at calculated positions and refined with a riding model. Final wR(F2) = 0.0691 and R(F) = 0.0358 for all data (388 refined parameters), GOF = 1.023, residuals Δρmin,max = −0.447, 0.430 e Å3. CCDC 195715. 30(2) K after irradiation: a = 8.461(2) Å, b = 8.370(2) Å, c = 18.759(4) Å, β = 97.15(3)°, V = 1318.1(5) Å3, Dc = 1.640 Mg m−3, μ = 0.663 mm−1, λ(Mo-Kα) = 0.71073 Å. Selected crystal mounted, 0.24 × 0.20 × 0.12 mm, and cooled to 30(2) K, data collected as above after irradiation for 30 min with an He–Ne laser (λ = 632.8 nm, 15 mW). Structure solution and refinement methods as above. 11729 reflections collected (Rint = 0.0281), 5269 unique. Final wR(F2) = 0.0759, R(F) = 0.0310 for all data (388 refined parameters), GOF = 1.058, residuals Δρmin,max = −0.639, 0.582 e Å3. CCDC 195714. See http://www.rsc.org/suppdata/cc/b2/b210146g/ for crystallographic data in CIF or other electronic format. Variable temperature powder X-ray diffraction: data were collected using a Bruker AXS D8 Advance diffractometer equipped with an Oxford Cryosystems PHENIX helium cryostat15 and a Braun linear PSD. A total of 24 patterns were collected between 30 and 300 K, with a heating rate of 17 K h−1, in the 2θ range between 5 and 35° in 0.0144° steps, using a counting time of 0.85 s/step, resulting in a collection time of 30 minutes per range. The model obtained from single crystal data was used to carry out Rietveld refinements of the 24 powder patterns. The only structural parameters refined were the cell parameters and an overall isotropic temperature factor. The other parameters refined were sample displacement, six background terms and six terms describing a pseudo Voigt profile function. |
| This journal is © The Royal Society of Chemistry 2003 |
