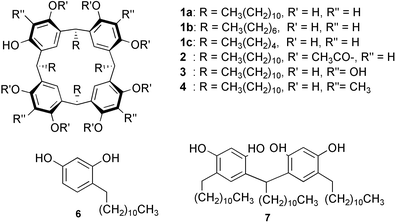
Chiral information transfer by solid–solid interaction: application for absolute configuration assignment†
Yasutaka
Tanaka
*,
Yoshinobu
Murakami
and
Riemi
Kiko
Department of Materials Science, Shizuoka University, Hamamatsu, Shizuoka, Japan. E-mail: tcytana@ipc.shizuoka.ac.jp; Fax: +81 53 478 1199; Tel: +81 53 478 1164
First published on 5th December 2002
Abstract
Host–guest complexes of calix[4]resorcarene with chiral molecules were efficiently formed by solid–solid grinding and exhibited CD Cotton effects reflecting the absolute configuration of the guest.
Calixarenes are macrocycles capable of accommodating a rather small molecule as a guest and thus are widely utilized as host molecules.1 The host–guest complexation involving calixarenes generally takes place in solution, thus allowing the process to be followed by means of solution spectroscopy such as NMR, absorption, and emission spectra. Herein we report that calix[4]resorcarene (1) efficiently forms a host–guest complex with a polar molecule by solid–solid reaction.2,3 If the guest is chiral, solid-state circular dichroism (CD) spectra of the complexes exhibit induced Cotton effects reflecting stereochemistry of chiral guests.4,5 The solid-state complexation and subsequent CD measurement is therefore applicable to predicting the absolute configuration of chiral guest molecules.
We have previously demonstrated that 1 in apolar organic solvents could form a 1∶1 complex with a polar molecule, such as carboxylic acids and sugars, via hydrogen-bonding interactions.6 The affinity of host 1 for the guests varied widely depending on the guest molecules: carboxylic acids and sugars were strongly (Ka = 103–105/l mol−1) and moderately (Ka = 101/l mol−1) bound,6 while aminoacids were scarcely bound to 1. Here we have found that 1 forms host–guest complexes by solid-state grinding when, in a typical example, 2.8 mg (2.5 × 10−3 mmol) of 1a and an equimolar amount of a guest were ground in an agate pestle and mortar for a few minutes. Powder X-ray diffraction patterns of the original 1a and guest disappeared after grinding together whereas those of 1a and guest were virtually invariant after grinding independently. The IR spectra of the ground mixture of 1a and a di- or mono-carboxylic acid guest, such as glutaric, 2-methylglutaric, and 2-phenylbutyric acids in a Nujol mull or KBr pellet gave a νOH signal at 3193 cm−1 for 1a and a νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1713 cm−1 for glutaric acid, whereas before grinding the values were at 3372 and 1730 cm−1, respectively, indicating that hydrogen bond formation between OH groups in 1a and guest carboxyl groups occurred.‡ The hydrogen bond formation was also confirmed by solid-state 13C NMR spectra.§ A ground mixture of 1a and methyl-β-D-glucopyranoside or aminoacids readily dissolves in CDCl3 and exhibits a typical 1H NMR spectrum of the 1a·guest complex: for example, the proton resonances from the glycoside C–H and O–H are largely shifted to high fields (Δδ
∼ 3 ppm) due to the ring current of the aromatic rings in 1a.7¶ On the other hand, the mixture without grinding is not completely soluble in CDCl3 and only shows the proton resonances of 1a. These spectroscopic results indicate that the complexation of 1a with guests through hydrogen bonding interactions took place by solid-state grinding and the complex structures were identical to those of the 1·guest complexes from solution.8|| Complexes were easily formed from 1a and an equal or slight excess (1–10 fold) of a variety of carboxylic acids, alcohols, amino acids, and even esters, aldehydes, and ketones, some of which were negligibly bound to 1 in solution. These results indicate complex formation occurred much more efficiently in the solid-state than in solution.
O at 1713 cm−1 for glutaric acid, whereas before grinding the values were at 3372 and 1730 cm−1, respectively, indicating that hydrogen bond formation between OH groups in 1a and guest carboxyl groups occurred.‡ The hydrogen bond formation was also confirmed by solid-state 13C NMR spectra.§ A ground mixture of 1a and methyl-β-D-glucopyranoside or aminoacids readily dissolves in CDCl3 and exhibits a typical 1H NMR spectrum of the 1a·guest complex: for example, the proton resonances from the glycoside C–H and O–H are largely shifted to high fields (Δδ
∼ 3 ppm) due to the ring current of the aromatic rings in 1a.7¶ On the other hand, the mixture without grinding is not completely soluble in CDCl3 and only shows the proton resonances of 1a. These spectroscopic results indicate that the complexation of 1a with guests through hydrogen bonding interactions took place by solid-state grinding and the complex structures were identical to those of the 1·guest complexes from solution.8|| Complexes were easily formed from 1a and an equal or slight excess (1–10 fold) of a variety of carboxylic acids, alcohols, amino acids, and even esters, aldehydes, and ketones, some of which were negligibly bound to 1 in solution. These results indicate complex formation occurred much more efficiently in the solid-state than in solution.
When a guest was chiral the solid-state circular dichroism spectrum (CD) of the complex exhibited induced CD possessing split Cotton effects at the absorption band of aromatic rings of 1a.9 The sample preparation for CD measurement is simple: the complex (0.1–0.2 mg) obtained by solid-state grinding was suspended in liquid paraffin (10 mg) and the suspension obtained was pasted onto a KBr plate or was injected into a NaCl liquid cell. 1aand the chiral guests themselves are otherwise CD silent in this region since 1a is inherently achiral and the guests are non-chromophores. For example, the CD of 1a·(R)- and 1a·(S)-2-phenylbutyric acid complexes are illustrated in Fig. 1, the enantiomeric complexes giving a pair of spectra which are mirror images of each other. Upon complexation of (S)-2-phenylbutyric acid to 1a, it is possible that the two adjacent benzene rings in 1a make a clockwise movement from front to back as shown in p-8 in Fig. 2 due to steric repulsion between the right benzene ring and the phenyl group as the larger substituent. In accordance with exciton-coupled circular dichroism, the clockwise orientation of the two equivalent benzene rings (positive chirality) gives rise to the first half (the longer wavelength half) of the split Cotton effect curve positive and second half negative.10 Thus, the positive or negative first Cotton effect was observed when the larger substituent was situated on the right or left, respectively, with respect to the stereogenic center such as in p-8 and n-8. The relationship between the stereochemistry of the chiral guests and the signs of induced CD are interpreted in the same way and they are highly correlated (Table 1). When the two substituents in a chiral guest possessed an identical size, such as CH3 and NH2 (in Table 1, L- and D-alanine) the chiral information transfer failed to provide an induced CD. The generality of the observations is suggestive of a rule which is practically applicable to the determination of absolute configurations.
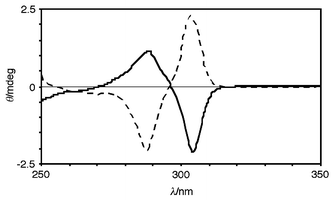 | ||
| Fig. 1 Solid-state CD spectra of 1a·R- (solid line) and 1a·S-2-phenylbutyric acid (dotted line) complexes in a Nujol mull. | ||
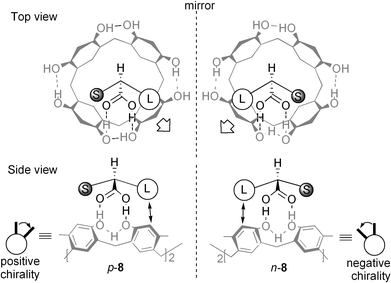 | ||
| Fig. 2 Schematic representation of chiral induction in 1 (gray drawing) upon complexation with S- and R-2-phenylbutyric acids (black drawing), in which L and S stand for the larger (phenyl) and smaller (ethyl) substituents. | ||
| Substituents | Cotton effect | |||
|---|---|---|---|---|
| Guest | Lefta | Righta | First | Second |
| a ‘Left’ and ‘right’ stand for the position of the larger (L) or smaller (S) substituents with respect to the stereogenic center in 8. b ‘nd’ indicates ‘not detected’. c D- and L-amino acids include 2-phenylglycine, valine, phenylalanine, leucine, isoleucine, and methionine. | ||||
| (S)-2-methylbutyric acid | ethyl (S)a | phenyl (L)a | + | − |
| (R)-2-methylbutyric acid | phenyl (L) | ethyl (S) | − | + |
| (S)-2-(6-methoxy-2-naphthyl)propionic acid | methyl (S) | naphthyl (L) | + | − |
| (S)-mandelic acid | hydroxyl (S) | phenyl (L) | + | − |
| (R)-mandelic acid | phenyl (L) | hydroxyl (S) | − | + |
| L-alanine | amino | methyl | ndb | ndb |
| D-alanine | methyl amino | ndb | ndb | |
| L-amino acidsc | amino (S) | residue (L) | + | − |
| D-amino acidsc | residue (L) | amino (S) | − | + |
| L-phenylalanine methyl-ester hydrochloride | amino (S) | benzyl (L) | + | − |
| L-valine methyl-ester hydrochloride | amino (S) | iso-propyl (L) | + | − |
| (S)-2-butanol | methyl (S) | ethyl (L) | + | − |
| (R)-1-phenyl-1-butanol | phenyl (S) | propyl (L) | + | − |
| (S)-1-phenyl-1-butanol | propyl (L) | phenyl (S) | − | + |
| (S)-2-(6-methoxy-2-naphthyl)propanol | methyl (S) | naphthyl (L) | + | − |
| (S)-2-(6-methoxy-2-naphthyl)propanal | methyl (S) | naphthyl (L) | + | − |
Multiple hydrogen-bond binding points (OH groups) having the correct orientation are essential features of the solid-state host. In fact, the octaacetate (2) of 1a, which has no OH groups, and calix[4]arene (5), where OH groups adopt an unfavorable orientation, did not function as a solid-state host despite having an aromatic cavity identical to 1a. On the other hand, the aromatic cavity in 1a is also an important structural aspect of the host: thus, complexation did not proceed when alkylresorcinol (6) and its dimer (7), which are constituents of 1a but lack the aromatic cavity, were used as a host. The length of the alkyl chains in 1, however, did not affect the binding ability since 1b and 1c also form complexes with the guests reported here. When 3 or 4 were adopted as the host instead of 1a, no or quite weak induced CD were observed probably because the extra four hydroxyl and methyl groups in 3 and 4 inhibited the guest insertion into the cavity.¶ The mechanism of efficient complexation here must be similar to that of the solid reaction recently reported: the intervention of a liquid phase has been claimed to explain the mechanism of an apparent solid–solid reaction.3 Thus, it is also possible here that a liquid or melt phase is induced as a result of a eutectic or peritectic melt phase occurring at the surface of the host or guest solids by grinding and that the host–guest complexation takes place consecutively in the liquid phase.
The complex formation of 1 with guest molecules by solid–solid grinding allows high efficiency of host–guest complexation and the use of a large excess of chiral guests as well as toxic organic solvents becomes unnecessary. The absolute configuration of the bound guest can be elucidated by solid-state CD measurement from the simple relationship of the signs of the induced CD.11
Notes and references
- C. D. Gutsche, Calixarenes Revisited, Royal Society of Chemistry, Cambridge, 1998 Search PubMed; Calixarenes 2001, ed. Z. Asfari, V. Böhmer, J. Harrowfield and J. Vicens, Kluwer, Dordrecht, 2001 Search PubMed.
- For the host–guest complexation by solid-state grinding, see: F. Toda, K. Tanaka and A. Sekikawa, J. Chem. Soc., Chem. Commun., 1987, 279 Search PubMed.
- For the covalent bond formation by solid-state grinding, see: G. W. V. Cave, C. L. Raston and J. L. Scott, Chem. Commun., 2001, 2159 Search PubMed.
- Co-crystals from solution exhibited induced CD in the solid-state, see: K. Tanaka, M. Kato and F. Toda, Chirality, 2001, 13, 347 Search PubMed.
- For absolute configuration assignments by induced CD due to intermolecular chiral information transfer in solution, see: Y. Kikuchi, K. Kobayashi and Y. Aoyama, J. Am. Chem. Soc., 1992, 114, 1351 Search PubMed; T. Morozumi and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 1219 CrossRef CAS; T. Kurtán, N. Nesnas, Y.-Q. Li, X. Huang, K. Nakanishi and N. Berova, J. Am. Chem. Soc., 2001, 123, 5974 RSC; H. Tsukube, M. Hosokubo, M. Wada, S. Shinoda and H. Tamiaki, Inorg. Chem., 2001, 40, 740 CrossRef CAS.
- Y. Aoyama, Y. Tanaka and S. Sugahara, J. Am. Chem. Soc., 1989, 111, 5397 CrossRef CAS; Y. Tanaka, Y. Kato and Y. Aoyama, J. Am. Chem. Soc., 1990, 112, 2807 CrossRef CAS.
- The 1H NMR spectrum of the ground mixture of 1a and methyl-β-D-glucopyranoside in CDCl3 is identical to that of the authentic complex, see: Y. Kikuchi, Y. Tanaka, S. Sutarto, K. Kobayashi, H. Toi and Y. Aoyama, J. Am. Chem. Soc., 1992, 114, 10302 Search PubMed.
- Y. Kikuchi, Y. Kato, Y. Tanaka, H. Toi and Y. Aoyama, J. Am. Chem., Soc., 1991, 113, 1349 CrossRef CAS.
- The solid-state CD has long been studied, see: R. Kuroda and Y. Saito, Bull. Chem. Soc. Jpn., 1976, 49, 433 Search PubMed.
- N. Harada and K. Nakanishi, Circular Dichroic Spectroscopy-Exciton Coupling in Organic Stereochemistry, University Science Books, Mill Valley, CA, 1983 Search PubMed.
- V. V. Borovkov, T. Harada, Y. Inoue and R. Kuroda, Angew. Chem., Int. Ed., 2002, 41, 1378 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: IR and 1H NMR spectra. See http://www.rsc.org/suppdata/cc/b2/b209281f/ |
‡ The authentic 1∶1 complex of 1a and glutaric acid from a CHCl3 solution for a KBr pellet also indicated a νOH in 1a at 3208 cm−1 and a νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic acid at 1714 cm−1. O in carboxylic acid at 1714 cm−1. |
§ The solid-state 13C NMR spectra (CPMAS, 67.5 MHz) showed a downfield shifted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbon resonance for bound glutaric acid at 179.9 ppm (182.3 ppm without 1a) for both the ground mixture of 1a and glutaric acid and the authentic 1∶1 complex of 1a and glutaric acid. O carbon resonance for bound glutaric acid at 179.9 ppm (182.3 ppm without 1a) for both the ground mixture of 1a and glutaric acid and the authentic 1∶1 complex of 1a and glutaric acid. |
| ¶ The experimental results also indicated that the solid-state complexation was a result of guest insertion into the host cavity rather than intercalation (clathrate formation) of guests into the 1a crystal lattice. For details of clathrate formation of calixarenes, see reference 1. |
| || The yields of the complexes based on 1a were estimated from quantitative IR and CD analyses. Calibration curves for the efficiency were obtained from the authentic 1∶1 1a·guest complexes from CHCl3 solution: 1a·dicarboxylic acid complexes (quant.), 1a·monocarboxlic acids (∼50%), 1a·monools (∼10%), and 1a·aminoacid complexes (∼5%). All complexes have a melting point of >300 °C. |
| This journal is © The Royal Society of Chemistry 2003 |