Stabilization of the previously unknown tautomer HP(OH)2 of hypophosphorous acid as ligand; preparation of [W3(Ni(HP(OH)2))Q4(H2O)9]4+(Q = S, Se) complexes†
Maxim N.
Sokolov
*a,
Rita
Hernández-Molina
b,
William
Clegg
c,
Vladimir P.
Fedin
a and
Alfredo
Mederos
b
aInstitute of Inorganic Chemistry SB RAS, pr. Lavrentyeva 3, 630090, Novosibirsk, Russia. E-mail: cluster@che.nsk.su; Fax: (+7)3832-344489
bDepartment of Inorganic Chemistry, University of La Laguna, 38200 La Laguna, Tenerife, Spain
cSchool of Natural Sciences (Chemistry), University of Newcastle upon Tyne, Newcastle upon Tyne, UK NE1 7RU
First published on 2nd December 2002
Abstract
Bis(hydroxy)phosphine, the isomer of hypophosphorous acid which remained elusive for a long time, coordinates to the Ni site of heterometallic clusters with a W3NiQ4 core (Q = S, Se) to give [W3(Ni(HP(OH)2))Q4(H2O)9]4+ (Q = S, Se).
Hypophosphorous acid (systematic name phosphinic acid) has a tetrahedral structure [H2P(O)(OH)] with two hydrogen atoms attached directly to phosphorus.1a It acts therefore only as a strong monobasic acid (pKa = 1.244), giving hypophosphites [H2PO2]−.1b The anion acts as a ligand via its oxygen atoms, while the phosphorus atom is devoid of donor properties, lacking a lone pair.2 The acid can also be protonated via its terminal oxygen to give [H2P(OH)2]+ (K ≈ 0.02).1c The tautomeric form [HP(OH)2] (see eqn. 1) has never been isolated or observed directly, though its presence in equilibrium with the [H2P(O)(OH)] tautomer has been repeatedly postulated from kinetics studies for a long time. It was estimated that the ratio [HP(OH)2]/[H2P(O)(OH)] in the equilibrium in aqueous solutions does not exceed 10−12.3,4 The three-coordinate [HP(OH)2] intermediate was also postulated in the reaction of NaH2PO2 with ROH (R = Me, Bu) in the presence of Pd catalyst, which leads to monoalkylphosphites HP(O)(OR)(OH) and H2 as the products.5 The cross-coupling of hypophosphites with ArX (X = Cl, Br, I) in the presence of Pd(PPh3)4 gives monoarylphosphinic acids ArP(H)(O)(OH). It is believed that the acids are formed from the [Ar–Pd–P(H)(OH)2]+ intermediate as the result of reductive elimination.6
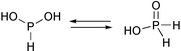
| (1) |
Recently we have shown that the elusive tautomer of phosphorous acid, the [P(OH)3] molecule, can be trapped and stabilized via coordination to the unique Pd site of the [Mo3(PdCl)S4(H2O)9]3+ cluster aqua ion.7 This is remarkable, since the uncoordinated tris(hydroxy)phosphine tautomer [P(OH)3] is thermodynamically unstable with respect to the [HP(O)(OH)2] form; log K = 10.3(1.5) at 25 °C in aqueous solution.8 In the present work we report the isomerization of [H2P(O)(OH)] into [HP(OH)2], which takes place in the presence of W3NiQ44+ (Q = S, Se) cuboidal clusters. By reaction of a new cluster [W3(NiCl)Se4(H2O)9]3+ (1)9 with H3PO2 in HCl, the bis(hydroxy)phosphine HP(OH)2 was trapped by coordination at the Ni site and, after addition of cucurbit[6]uril, the complex was isolated12 and structurally characterized as a supramolecular adduct [W3(Ni(HP(OH)2))Se4(H2O)9]Cl4·C36H36N24O12·11H2O (2).13 The sulfur analogue of 1 gives isomorphous crystals of [W3(Ni(HP(OH)2))S4(H2O)9]Cl4·C36H36N24O12·11H2O (3) under the same conditions.14
The formation of the [W3(Ni(HP(OH)2))Q4(H2O)9]4+ complexes (Q = S, Se) in solution is relatively slow, but quantitative, and can be followed by UV-Vis spectroscopy.15 The products are rather stable and can be eluted from a Dowex cation exchange column with 1–4 M HCl without any decomposition. In the case of [W3(Ni(HP(OH)2))Se4(H2O)9]4+ the 31P NMR spectrum of the eluate in 2 M HCl shows the expected doublet, which turns into a sharp singlet (129.0 ppm from 85% H3PO4) when the P–H coupling is suppressed. The observed value of 1JP–H is 392.8 Hz, which can be compared with 180–225 Hz for three-coordinate ![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) P–H systems16 as well as with 575.9 Hz for [H2P(O)(OH)] and with 686.0 Hz for [HP(O)(OH)2]1c The reactions with H3PO2 take about 1 h to complete (at mM levels).
P–H systems16 as well as with 575.9 Hz for [H2P(O)(OH)] and with 686.0 Hz for [HP(O)(OH)2]1c The reactions with H3PO2 take about 1 h to complete (at mM levels).
The crystallization of 2, 3 in high yields is achieved by adding cucurbit[6]uril to the reaction mixture. This macrocyclic cavitand forms stable adducts with [M3Q4(H2O)9]4+ (M = Mo, W; Q = S, Se) incomplete cube clusters, and with their heterometal cuboidal derivatives, such as [Mo3(NiCl)S4(H2O)6Cl3]+.17,18 The driving force is the formation of twelve complementary hydrogen bonds between the C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O groups of cucurbituril and water molecules coordinated to the cluster. Each W atom in 2 is coordinated by three water molecules (Fig. 1). Coordinated water molecules form complementary hydrogen bonds with the portal oxygen atoms of cucurbituril, with O⋯O distances in the range 2.610–2.845 Å, although the hydrogen atoms could not be unambiguously located by crystallography. The ligand [HP(OH)2] is bound to Ni via the phosphorus atom (Ni–P 2.128(4) Å). This bond is rather short. In the cluster [Fe3(NiPPh3)S4(SEt)3] the Ni–P bond is 2.18 Å,19 and in [Ni4(μ-CO)6(P(CH2CH2CN)3)4] it is 2.16 Å.20 The P–O distances in 2 are 1.585(11) and 1.622(12) Å. In [Mo3(Pd(P(OH)3))S4Cl3(H2O)6]+ a shorter P–O bond distance of 1.561 Å is found. The P–H hydrogen atom in 2 could be located only tentatively in a difference Fourier synthesis. Our formulation is, however, strongly supported by the 31P NMR data in solution (purified by column chromatography), which clearly show the presence of a species with only one direct P–H bond. No other signals were observed. The angles O–P–Ni are 116.2(5) and 116.4(5)°, and O–P–O is 105.2(7)°, which in sum give 337.8°, much less than 360° for trigonal planar P, but only 9° more than the sum of three tetrahedral angles. The refined position of the H atom bonded to P, although it has large uncertainties, is consistent with a pyramidal phosphorus atom.
O groups of cucurbituril and water molecules coordinated to the cluster. Each W atom in 2 is coordinated by three water molecules (Fig. 1). Coordinated water molecules form complementary hydrogen bonds with the portal oxygen atoms of cucurbituril, with O⋯O distances in the range 2.610–2.845 Å, although the hydrogen atoms could not be unambiguously located by crystallography. The ligand [HP(OH)2] is bound to Ni via the phosphorus atom (Ni–P 2.128(4) Å). This bond is rather short. In the cluster [Fe3(NiPPh3)S4(SEt)3] the Ni–P bond is 2.18 Å,19 and in [Ni4(μ-CO)6(P(CH2CH2CN)3)4] it is 2.16 Å.20 The P–O distances in 2 are 1.585(11) and 1.622(12) Å. In [Mo3(Pd(P(OH)3))S4Cl3(H2O)6]+ a shorter P–O bond distance of 1.561 Å is found. The P–H hydrogen atom in 2 could be located only tentatively in a difference Fourier synthesis. Our formulation is, however, strongly supported by the 31P NMR data in solution (purified by column chromatography), which clearly show the presence of a species with only one direct P–H bond. No other signals were observed. The angles O–P–Ni are 116.2(5) and 116.4(5)°, and O–P–O is 105.2(7)°, which in sum give 337.8°, much less than 360° for trigonal planar P, but only 9° more than the sum of three tetrahedral angles. The refined position of the H atom bonded to P, although it has large uncertainties, is consistent with a pyramidal phosphorus atom.
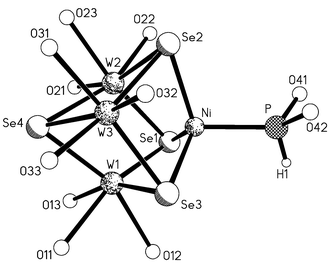 | ||
| Fig. 1 The structure of the cation in 2. Selected bond lengths (Å): W–Se 2.4732(11)–2.4851(11), W–O 2.151(7)–2.308(8), Ni–Se 2.3053(17)–2.3089(18), Ni–P 2.128(4), P–O 1.585(11) and 1.622(12). | ||
The behaviour of Ni in the clusters W3NiQ4 (Q = S, Se) indicates a high degree of softness. The ability to induce the isomerization of [H2P(O)(OH)] shows that the formation of one short and strong Ni–P bond compensates the unfavourable energetic changes in the rearrangement of [H2P(O)(OH)] into [HP(OH)2]. It was also reported that [W3NiS4(H2O)10]4+ binds C2H4 and CO at the Ni site.11,21 This is the more important, as the only other available Ni aqua species, the aqua ion [Ni(H2O)6]2+, behaves totally differently in this respect. Ni(II) hypophosphite exists as NiCl(H2PO2)·H2O, where tetrahedral H2PO2− bridges two [NiO3Cl2(H2O)] units through its oxygen atoms.22 This unusual reactivity at the Ni site deserves to be further explored end exploited for the design of structural and functional models for bioclusters and transition metal sulfide-based hydroprocessing catalysts. Indeed, the Mo/Ni cluster [Mo3NiS4(H2O)10]4+, incorporated into zeolites, gives efficient catalysts for benzothiophene hydrodesulfurization,23 as well as highly selective catalysts for the formation of C2 species from CO and H2.24 Ni is also essential in several bacterial enzymes, and Ni sites there are often the place of highly unusual reactions (from the point of view of ‘normal’ Ni (II) chemistry). The enzyme CO dehydrogenase is believed to contain a cubane cluster core Fe3NiS4.25
This work has been supported by the Spanish Ministry of Education and Culture (grant to M. N. S. within the program ‘Stay of young scientists and technologists in Spain’), by the University of La Laguna (an invited professor grant to V. P. F.), and by EPSRC (UK; equipment grant to W. C.). INTAS support for M. N. S. and V. P. F. is gratefully acknowledged.
Notes and references
- (a) N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworths-Heinemann, Oxford, 2nd edn., 1998, pp. 513–514 Search PubMed; (b) J. W. Larson and H. Pippin, Polyhedron, 1989, 8, 527 CrossRef CAS; (c) T. E. Haas and H. G. Gillmann, Inorg. Chem., 1968, 7, 2051 CrossRef CAS.
- Structurally characterized are salts/complexes of NH4+, K+, Ca2+, Mg2+, Zn2+, U4+, Co2+, Ni2+, Mn2+, Cu2+ Sn2+, Ge2+, Ln3+, UO22+. The relevant references can be found in: (a) P. A. Tanner, M. D. Faucher and T. C. W. Mak, Inorg. Chem., 1999, 38, 6008 CrossRef CAS; (b) P. A. Tanner and T. C. W. Mak, Inorg. Chem., 1999, 38, 6024 CrossRef CAS; (c) D. Yu. Naumov, D. S. Yufit, E. V. Boldyreva and J. A. K. Howard, Acta Crystallogr., Sect. C, 2001, 57, 790 CrossRef CAS.
- J. R. Van Wazer, Phosphorus and Its Compounds, Interscience Publishers, Inc., New York, 1958, vol. 1, pp. 364–367 Search PubMed.
- W. A. Jenkins and D. M. Yost, J. Inorg. Nucl. Chem., 1959, 11, 297 CrossRef CAS.
- Y. A Dorfman and M. M. Aleshkova, Kinet. Catal., 1998, 39, 852 Search PubMed.
- J.-L. Montchamp and Y. R. Dumond, J. Am. Chem. Soc., 2001, 123, 510 CrossRef CAS.
- M. N. Sokolov, A. V. Virovets, D. N. Dybtsev, E. V. Chubarova, V. P. Fedin and D. Fenske, Inorg. Chem., 2001, 40, 4816 CrossRef CAS.
- J. P. Guthrie, Can. J. Chem., 1979, 57, 236 CAS.
- Dark-green [W3(NiCl)Se4(H2O)9]3+ (1) was prepared by reacting metallic Ni with [W3Se4(H2O)9]4+10 in 2 M HCl (18 h, 90 °C, yield 70%) in a manner analogous to that for [W3NiS4(H2O)10]4+.11 It exists as the monomeric species [W3(NiCl)Se4(H2O)9]3+ in 2 M HCl (electronic spectrum, λ/nm (ε/mol−1 cm−1) 753(590), 633(480), 457(860)) and as [W3NiSe4(H2O)10]4+ in 2 M Hpts (p-toluenesulfonic acid) (electronic spectrum, λ/nm (ε/mol−1 cm−1) 746(554), 627(542), 446(1007)). From a solution in 3 M Hpts it crystallizes as the edge-linked double cube cluster {W3NiSe4(H2O)9}2(pts)8·18H2O, the structure of which will be discussed elsewhere.
- V. P. Fedin, M. N. Sokolov, A. V. Virovets, N. V. Podberezskaya and V. E. Fedorov, Inorg. Chim. Acta, 1998, 269, 292 CrossRef CAS.
- T. Shibahara, G. Sakane, M. Maeyama, H. Kobashi, T. Yamamoto and T. Watase, Inorg. Chim. Acta, 1996, 251, 207 CrossRef CAS.
- [W3(Ni(HP(OH)2))Se4(H2O)9]Cl4·C36H36N24O12·11H2O (2). 1 ml of a 5 mM solution of 1 in 1 M HCl was mixed with 1 ml of saturated cucurbituril solution in 4 M HCl, and then 1 drop of H3PO2 (50% w/w aqueous solution) was added. The vial was closed under nitrogen and left overnight. Well-formed rectangular red–brown crystals were collected..
- Crystal data for 2: C36H77Cl4N24NiO34PSe4W3, Mr = 2489.1, monoclinic, space group C2/c, a = 16.5728(5), b = 18.5868(6), c = 48.7091(16) Å, β = 99.391(2)°, V = 14803.0(8) Å3, Z = 8, ρcalc = 2.234 g cm−3, MoKα radiation, λ = 0.71073 Å, μ = 7.13 mm−1, T = 160 K. 51489 measured reflections were corrected for absorption, 13020 were unique (Rint = 0.049, θ <25°); R = 0.060 (F values, F2 > 2σ, Rw = 0.132 (F2 values, all data), GOF = 1.18 for 977 parameters; H atoms were included with a riding model for the cucurbituril molecules, and without geometrical constraints for H1 bonded to P, but they were not located or included on water molecules. Minor disorder was resolved for some uncoordinated water molecules, and may be present, unresolved, in others and for the chloride anions, around which the largest difference electron density peaks are found. CCDC 179026. See http://www.rsc.org/suppdata/cc/b2/b209792c/ for crystallographic data in CIF or other electronic format.
- [W3(Ni(HP(OH)2))S4(H2O)9]Cl4·C36H36N24O12·11H2O (3) was prepared analogously, using [W3(NiCl)S4(H2O)9]3+.11 Cell parameters (C2/c): a = 16.4898(8), b = 18.7332(9), c = 48.397(2) Å, β = 97.620(2)° Å3.
- Electronic spectra for [W3(Ni(HP(OH)2))Se4(H2O)9]4+ (2 M Hpts, λ/nm (ε/mol−1 cm−1): 520(790), 442(1240).
- Encyclopedia of Inorganic Chemistry, ed. R.B. King, vol. 6, John Wiley & Sons, Chichester, 1994, p. 3157 Search PubMed.
- R. Hernández-Molina, M. N. Sokolov and A. G. Sykes, Acc. Chem. Res., 2001, 34, 223 CrossRef CAS.
- V. P. Fedin, V. Gramlich, M. Wörle and T. Weber, Inorg. Chem., 2001, 40, 1074 CrossRef CAS.
- S. Ciurli, S.-B. Yu, R. H. Holm, K. K. P. Shrivastava and E. Münck, J. Am. Chem. Soc., 1990, 112, 8169 CrossRef CAS.
- M. A. Bennett, F. A. Cotton and B. H. C. Winquist, J. Am. Chem. Soc., 1967, 89, 5366 CrossRef CAS.
- I. Schmidt, J. Hyldtoft, J. Hjortkær and C. J. H. Jacobsen, Acta Chem. Scand., 1996, 50, 871 Search PubMed.
- M. D. Marcos, P. Amorós, F. Sapiña, A. Beltran-Porter, R. Martinez-Mañez and J. P. Attfield, Inorg. Chem., 1993, 32, 5044 CrossRef CAS.
- M. Taniguchi, D. Imamura, H. Ishige, Y. Ishii, T. Murata, M. Hidai and T. Tatsumi, J. Catal., 1999, 187, 139 CrossRef CAS.
- M. Taniguchi, Y. Ishii, T. Murata, T. Tatsumi and M. Hidai, J. Chem. Soc., Chem. Commun., 1995, 2533 RSC.
- M. A. Halcrow and G. Christou, Chem. Rev., 1994, 94, 2421 CrossRef CAS.
Footnote |
| † Dedicated to Professor Achim Müller on the occasion of his 65th birthday. |
| This journal is © The Royal Society of Chemistry 2003 |
