Synthesis and fluorescence enhancement of oligophenylene-substituted calix[4]arene assemblies†
Man Shing
Wong
*,
Xiao Ling
Zhang
,
Dong Zhong
Chen
and
Wai Ho
Cheung
Department of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong, S. A. R.. China. E-mail: mswong@hkbu.edu.hk
First published on 3rd December 2002
Abstract
Tetra-oligophenylene substituted calix[4]arene assemblies containing up to three phenylene units have been synthesized by a convergent approach using Suzuki cross-coupling and their optical properties were investigated and compared with the corresponding monomer.
Calixarenes1 have been widely exploited as supramolecular building blocks for molecular encapsulation2,3 as well as ion recognition and sensing4 in the last decade. Recently, it has been of great interest to extend the rigid cavity at the upper rim of calixarenes for expanded cavity receptors5 as they are beneficial to the encapsulation and recognition properties. In addition, the use of calix[4]arene as the pre-defined and pre-organized framework to incorporate with extended chromophores for intramolecular electronic transfer6 and inter-chromophoric interaction investigations7,8 has drawn some attention. As a result, there are considerable synthetic effort to expand the rigid cavity at the upper rim of calix[4]arenes. We are particularly interested in synthesizing oligophenylene substituted calixarene assemblies for functional property investigations as the highly extended styrylstyrylcalix[4]arene derivatives show poor photostability in solution which is presumably due to the photo-dimerization of the proximate co-facial vinyl units.
Several methods have been developed to prepare p-phenylcalix[4]arene;9 however, these procedures afford very low yields. On the other hand, p-phenylcalix[4]arene tetraalkyl ethers are easily synthesized in good yields by various metal catalysed cross-coupling protocols, which include Suzuki-type coupling,10,11 and Negishi-type coupling.12 Nevertheless, there is a lack of example or methodology of synthesizing longer homologues of oligophenylene substituted calix[4]arenes reported so far. To this end, we report herein a facile protocol to synthesize an homologues series of tetra-oligophenylene substituted calix[4]arene assemblies containing up to three phenylene units end-capped with alkylsulfanyl donating substituents, 9a–c and with alkylsulfonyl withdrawing substituents, 10a–c. In addition, their optical properties were characterized and compared with the corresponding monomer.
We found that Suzuki cross coupling was particularly versatile for the convergent synthesis of the homologues series of oligophenylene-substituted calix[4]arene assemblies as good reactivity and regioselectivity could be obtained by various catalytic palladium compounds/complexes. Cross coupling of oligoarylboronic acid and tetrahalocalix[4]arene tetraalkyl ether was used as a key step to construct the highly extended cone-conformed assemblies. The syntheses of (oligo-)arylboronic acids are summarized in Scheme 1. Alkylation of 4-bromothiophenol 1 with 1-bromohexane in basic medium afforded 1-bromo-4-hexylsulfanylbenzene in excellent yield (97%). Lithium bromide exchange of the bromine at −78 °C followed by quenching with trimethyl borate at room temperature and subsequent acid hydrolysis afforded 4-hexylsulfanylbenzeneboronic acid 2. 4-Bromo-4′-hexylsulfanylbiphenyl 4 was prepared either by regioselective Suzuki cross-coupling of 1-bromo-4-iodobenzene with 2 (50%) or by nucleophilic aromatic substitution of 4,4′-dibromobiphenyl 3 with 1-hexanethiol in the presence of NaOH in DMF (62%). Subsequent conversion of bromide to boronic acid functionalities afforded 4′-hexylsulfanyl-4-biphenylboronic acid 5 in a good yield. Hexylsulfanyl-substituted terphenylboronic acid 6 was prepared accordingly using the same stepwise approach as described above starting with the two-phenyl-ring boronic acid 5.
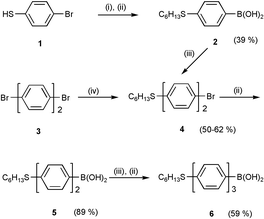 | ||
| Scheme 1 Reagents and conditions: (i) C6H13Br, NaH, DMF, 70 °C, overnight; (ii) a, BunLi, THF, −78 °C, 1 h. b, B(OMe)3, −78 °C to rt, 1 h. c, H+; (iii) IC6H4Br, 5 mol% Pd(OAc)2–2P(o-tol)3, K2CO3, toluene, 50 °C, 6 h; (iv) C6H13SH, NaOH, DMF, 150 °C. | ||
Tetraiodocalix[4]arene tetradecyl ether 7 was prepared according to the procedures published in the literature.13 Cross-coupling of 7 with boronic acid 2 or 5 in the presence of a catalytic amount of Pd(OAc)2–2P(o-tol)3 complex afforded the corresponding tetraoligophenylene substituted calix[4]arene assemblies 9a or 9b, respectively, in good yields (Scheme 2) It should be stated that the Pd(OAc)2–2P(o-tol)3 complex is found to be more efficient than the classical Pd(PPh3)4 as a catalyst for the cross-coupling and the coupling reaction also worked well with tetrabromocalix[4]arene tetradecyl ether but required more drastic conditions. However, the reaction of tetraiodocalix[4]arene 7 with terphenylboronic acid 6 could not be driven to completion which is presumably due to the increased steric crowdedness of the calix[4]arene reaction sites created by the assembled oligophenylene units particularly in the construction of the higher homologues of an assembly. To alleviate the crowdedness of the calix[4]arene reaction sites, the extended tetrabromophenylcalix[4]arene tetradecyl ether 8 was pursued and prepared by selective cross-coupling of commercially available bromophenylboronic acid and 7 using Pd(PPh3)4 as a catalyst. It is important to note that the use of Pd(OAc)2–2P(o-tol)3 the complex as a catalyst in this reaction afforded no desired product. Cross coupling of 8 and biphenylboronic acid 5 under typical conditions afforded 9c in a moderate yield. (Scheme 2). Besides, assembly 9b can be prepared by coupling of 8 with boronic acid 2. The MALDI-TOF mass spectra of 9b and 9c showed a major peak at m/z 2081 and 2385, respectively, corresponding to [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na]+. MCPBA-oxidation of alkylsulfanyl-substituted calix[4]arene assemblies, 9a–c in CH2Cl2 afforded the corresponding alkylsulfonyl-substituted calix[4]arene assemblies, 10a–c, respectively in moderate to good yields. The MALDI-TOF mass spectra of 10b and 10c showed a major peak at 2210 corresponding to [M
Na]+. MCPBA-oxidation of alkylsulfanyl-substituted calix[4]arene assemblies, 9a–c in CH2Cl2 afforded the corresponding alkylsulfonyl-substituted calix[4]arene assemblies, 10a–c, respectively in moderate to good yields. The MALDI-TOF mass spectra of 10b and 10c showed a major peak at 2210 corresponding to [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2H
2H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na]+ and 2512 corresponding to [M
Na]+ and 2512 corresponding to [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na]+, respectively. In addition to low/high resolution mass spectroscopy, all the newly synthesized assemblies were characterized with 1H NMR and 13C NMR and found to be in good agreement with expected structures.†
Na]+, respectively. In addition to low/high resolution mass spectroscopy, all the newly synthesized assemblies were characterized with 1H NMR and 13C NMR and found to be in good agreement with expected structures.†
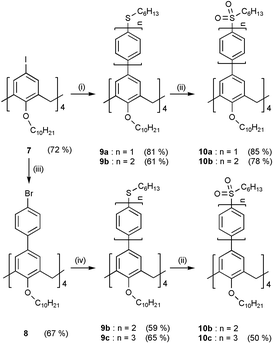 | ||
| Scheme 2 Reagents and conditions: (i) C6H13S(C6H4)nB(OH)2, 40 mol% Pd(OAc)2–2P(o-tol)3, K2CO3, toluene, 50 °C, 6 h; (ii) MCPBA, CH2Cl2, 0 °C, 1 h; (iii) BrC6H4B(OH)2, 40 mol% Pd(PPh3)4, K2CO3, toluene, 45 °C, overnight; (iv) C6H13S(C6H4)n−1B(OH)2, 40 mol% Pd(OAc)2–2P(o-tol)3, K2CO3, toluene, 50 °C, 6 h. | ||
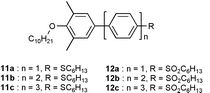
For comparison, the corresponding monomers 11a–c and 12a–c were also synthesized using the same convergent approach. Dependent on the nature of substituents end-capped on the oligophenylene skeletons, the optical properties of the assemblies vary differently with respect to the corresponding monomer. Even though the spectral features of the assemblies and the corresponding monomer are similar, the assemblies 9a–c bearing hexylsulfanyl donating groups exhibit a slightly blue shift in the absorption maxima, λmax and the assemblies bearing hexylsulfonyl withdrawing groups 10a–c consistently exhibit red shift relative to the corresponding monomer (Table 1) This suggest that oligophenylenes built onto the calix[4]arene framework show little interaction in the ground-state. In contrast to the absorption behaviour, the red shift of the emission maxima of the assemblies are substantial in both series as compared to those of the corresponding monomer. This implies that the excited state chromophoric interaction within an assembly is significant. On the other hand, the excitonic interaction only affects the fluorescence lifetimes (τ) of the assemblies in a lesser extent relative to the corresponding monomer. For the hexylsulfanyl end-functionalized assemblies 9b,c, the fluorescence quantum yields (ΦFL) are found to be substantially lower than that of the corresponding monomer 11b,c. It is consistent to the general observations that ordered packing/arrangement of the emissive molecules or polymers often leads to excimers formation and then fluorescence quenching. However, the hexylsulfonyl end-functionalized assemblies 10b,c consistently exhibit larger fluorescence quantum yields than that of the corresponding monomer 12b,c. This result implies that acentric arrangements of donor–acceptor fluorophores will lead to fluorescence enhancement.
| Compound | λ abs max a/nm |
104εmaxa/
M−1 cm−1 |
λ em max a/nm | Φ FL a , b | τ/nsa,c |
|---|---|---|---|---|---|
| a Measured in CHCl3. b Average of two independent measurements and using quinine in 1 M H2SO4 (Φ313 = 0.48 at 313 nm) as a standard. c Using nitrogen laser as an excitation source. | |||||
| 9a | 283 | 10.12 | 379 | 0.82 | |
| 9b | 301 | 14.89 | 384 | 0.12 | 0.84 |
| 9c | 312 | 22.65 | 386/398 | 0.31 | 0.85 |
| 10a | 284 | 6.99 | 373 | 1.02 | |
| 10b | 303 | 11.04 | 412 | 0.77 | 1.03 |
| 10c | 313 | 15.26 | 427 | 0.74 | 1.01 |
| 11a | 287 | 2.37 | 372 | 0.76 | |
| 11b | 307 | 3.47 | 375 | 0.28 | 0.78 |
| 11c | 315 | 5.07 | 379/390 | 0.51 | 0.80 |
| 12a | 282 | 1.91 | 366 | 1.34 | |
| 12b | 302 | 3.22 | 405 | 0.70 | 1.38 |
| 12c | 312 | 4.23 | 414 | 0.60 | 1.37 |
In summary, we have developed a convergent approach using Suzuki cross-coupling reaction to synthesize novel series of highly extended tetra-oligophenylene substituted calix[4]arene assemblies containing up to three phenylene units end-capped with hexylsulfanyl donating groups or hexylsulfonyl withdrawing groups. We have also found that, in contrast to the electron-donor substituted assemblies, the hexylsulfonyl end-functionalized assemblies exhibit fluorescence enhancement as compared to the corresponding monomers. These findings may provide a new avenue to design fluorophores and emissive materials for luminescence applications.
This work was supported by Faculty Research Grant (FRG/01-02/II-43) from Hong Kong Baptist University and Earmarked Research Grant (HKBU2019/02P) from Research Grants Council, Hong Kong. We gratefully acknowledge Dr Y. Tao at NRC, Canada for the MALDI-TOF mass spectrometry measurements.
Notes and references
- For recent review, see: Calixarenes 2001, ed. Z. Asfari, V. Bohmer, J. Harrowfield and J. Vicens, Kluwer, Dordrecht, 2001 Search PubMed.
- F. Hof, S. L. Craig, C. Nuckolls and J. Rebek, Angew. Chem., Int. Ed., 2002, 41, 1488 CrossRef CAS.
- J. Rebek, Chem. Commun., 2000, 637 RSC.
- A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713 CrossRef CAS.
- Y. L. Cho, D. M. Rudkevich and J. Rebek, J. Am. Chem. Soc., 2000, 122, 9868 CrossRef CAS.
- A. Harriman, M. Hissler, P. Jost, G. Wipff and R. Ziessel, J. Am. Chem. Soc., 1999, 121, 14 CrossRef CAS.
- M. S. Wong, Z. H. Li and C. C. Kwok, Tetrahedron Lett., 2000, 41, 5719 CrossRef CAS.
- T. Gu, G. Accorsi, N. Armaroli, D. Guillon and J.-F. Nierengarten, Tetrahedron Lett., 2001, 42, 2309 CrossRef CAS.
- M. Makha and C.-L. Raston, Tetrahedron Lett., 2001, 42, 6215 CrossRef CAS and references therein.
- M. S. Wong and J.-F. Nicoud, Tetrahedron Lett., 1993, 34, 8237 CrossRef CAS.
- R. K. Junejia, K. D. Robinson, C. P. Johnson and J. L. Atwood, J. Am. Chem. Soc., 1993, 115, 3818 CrossRef.
- M. Larsen and M. Jorgensen, J. Org. Chem., 1997, 62, 4171 CrossRef CAS.
- P. Timmerman, W. Verboom, D. N. Reinhoudt, A. Arduini, A. R. Sicuri, A. Pochini and R. Ungaro, Synthesis, 1994, 185 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: experimental procedures and spectral data of all new compounds. See http://www.rsc.org/suppdata/cc/b2/b210493h/ |
| This journal is © The Royal Society of Chemistry 2003 |