High acid catalytic activity of aluminosilicate molecular sieves with MCM-41 structure synthesized from precursors of colloidal faujasite†
Javier
Agúndez
,
Isabel
Díaz
,
Carlos
Márquez-Álvarez
,
Joaquín
Pérez-Pariente
* and
Enrique
Sastre
Instituto de Catálisis y Petroleoquímica, CSIC, Cantoblanco, 28049, Madrid, Spain. E-mail: jperez@icp.csic.es; Fax: 34 91585 4760; Tel: 34 91585 4784
First published on 4th December 2002
Abstract
The synthesis of micro/mesoporous aluminosilicate with a hexagonal arrangement of pores has been achieved by cationic surfactant-templating in a tetramethylammonium-containing solution precursor of colloidal faujasite; this material is zeolite nanocrystal-free and exhibits high catalytic activity in m-xylene conversion.
The low acidity and hence moderate catalytic activity of aluminium-containing ordered mesoporous materials, particularly Al-MCM-41, is generally attributed to the well-known absence of structural order in the aluminosilicate matrix.
A number of strategies have been reported in the last few years to increase the activity of mesoporous aluminosilicates in acid-catalyzed reactions.1–3 One of such approaches consists in mimicking the physicochemical conditions prevalent in the synthesis gels from which zeolite materials are obtained, and promoting the surfactant-assisted assembly of the zeolite precursors present in the gel. Mesostructures characterized by enhanced activity have been obtained from ZSM-5 and Beta seeds.2
The synthesis of ordered mesoporous materials possessing a faujasite-like framework would be particularly appealing, due to the importance of this zeolite in the oil refining industry. Steam-stable mesoporous materials have been synthesized through the assembly of Y zeolite seeds mediated by hexadecyltrimethylammonium (CTA) cations, but the activity that these aluminosilicates exhibited, before steaming, in the cracking of cumene was close to that of Al-MCM-41 synthesized by conventional methods.3 This process made use of a pure inorganic seeding gel, whereas tetraalkylammonium cations, TPA and TEA, are present in the synthesis gels from which mesoporous materials of enhanced catalytic activity are obtained.2 Therefore, it could be possible that the presence of organic cations in the zeolite gel precursor is required to obtain materials possessing high acid strength.
We report in this work the synthesis and catalytic activity of aluminosilicates with hexagonal pore arrangement from tetramethylammonium-containing solutions that are precursors of faujasite crystals of colloidal size.
The synthesis procedure involves the preparation of a solution precursor of colloidal faujasite, with the following molar composition:4
| Al2O3∶1.53 (TMA)2O∶0.088 Na2O∶3.62 SiO2∶246 H2O |
This solution was mixed at room temperature with 101.85 g of a 20 wt% hexadecyltrimethylammonium (CTA) bromide aqueous solution, and heated at selected temperatures for 3 h in polypropylene bottles (if T < 100 °C) or in Teflon-lined, stainless-steel 60 ml sized-autoclaves (if T ≥ 100 °C). The solid was then filtered off, washed with deionized water and dried at 60 °C overnight. The total yield of oxides averaged 60%. The samples were calcined at 550 °C under continuous flow of N2 (130 cm3 min−1) for 1 h, followed by air (130 cm3 min−1) for 6 h. For comparison purposes, two aluminium-rich Al-MCM-41 samples were prepared according to refs. 6 and 7.
The X-ray diffraction pattern of the as-made sample synthesized at 150 °C (Fig. 1) is characteristic of a hexagonal arrangement of pores with a unit cell size (a0) of 4.3 nm. No reflections at angles higher than that corresponding to the 210 reflection of the hexagonal symmetry are observed (see inset in Fig. 1). Upon calcination at 550 °C in N2/air flow, a single peak is observed in the XRD pattern (Fig. 1), shifted to a d value (d = 2.3 nm) much lower than that corresponding to the most intense reflection of the as-made material. We observed a decrease in intensity and the broadening of the XRD profile for samples synthesized at temperatures below 150 °C, whereas at 175 °C, well-ordered MCM-41-type materials are obtained.
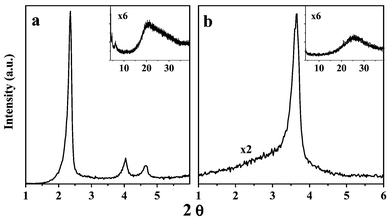 | ||
| Fig. 1 XRD patterns of sample synthesized at 150 °C, as-made (a) and calcined (b). | ||
TEM images of a calcined sample synthesized at 150 °C (Fig. 2) show the hexagonal array of channels characteristic of the MCM-41 structure. By Fast Fourier Transform (FFT) of the TEM images (Fig. 2, inset) we estimate a unit cell dimension of 2.9 nm, in good agreement with the 2.8 nm value obtained by XRD assuming that the peak at d = 2.3 nm corresponds to the 100 reflection of the hexagonal p6mm symmetry. Therefore, a unit cell contraction of ca. 1.4 nm takes place upon calcination. This severe shrinkage is not totally unexpected, taking into account that the as-made material contains as much as 49 wt% organic material, which suggests that it possesses a large amount of structural defects.
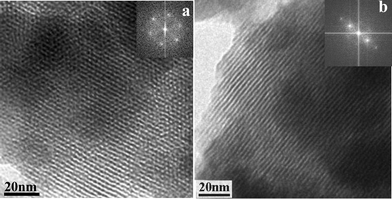 | ||
| Fig. 2 TEM images and corresponding FFT patterns along the direction parallel (a) and normal (b) to the channel axes in an MCM-41 type structure of the sample synthesized at 150 °C and calcined. | ||
The small unit cell suggests that the pore size of the material should be unusually small as well, and close to the border between micro- and mesopores (2.0 nm), in order to avoid unreasonable wall thickness. The small pore size is reflected in the N2 isotherm (ESI†), where the conventional, sharp step of pore filling at p/p0 values in the range 0.2–0.4 is not observed. Instead, microporosity (0.24 cm3 g−1) is detected by t-plot analysis of the N2 isotherm. However, the pore size distribution in the micropore region determined by Ar adsorption does not show evidence of faujasite-type micropores, whereas a weak, broad maximum centered at ca. 1.4 nm is present.
The 29Si MAS NMR spectrum of the calcined sample synthesized at 150 °C (Fig. 3) shows a broad peak centered at ca. −102 ppm where prominent shoulders are observed. Deconvolution of the spectrum suggests the presence of two different sets of silicon environments, one of them comprising four distinct Si locations centered at –107, –102 (the most intense one), −97 and −92 ppm. The chemical shifts and relative intensities of these signals are similar, respectively, to Si(0Al), Si(1Al), Si(2Al) and Si(3Al) species in a zeolite-like environment. The broad peaks centered at −110 and −93 ppm could be attributed to a different phase and might arise from amorphous silicate species. The 27Al MAS NMR spectrum of the same calcined sample (Fig. 3) shows the presence of three distinct signals centered at 1, 27 and 55 ppm. The high-field signal corresponds to octahedral aluminium, and that at 55 ppm is assigned to tetrahedral (structural) aluminium. Of interest is the presence of the peak at 27 ppm, which has been assigned to five-coordinate aluminium or, alternatively, aluminium in highly distorted tetrahedral sites in dealuminated faujasite.8 This so-called pentacoordinated aluminium is in general not observed in calcined aluminium-rich Al-MCM-41 materials even for very low Si/Al ratios,9 although it has been identified under certain synthesis conditions10
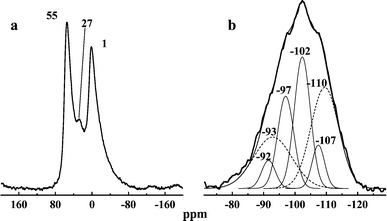 | ||
| Fig. 3 27Al (a) and 29Si (b) MAS NMR spectra of the calcined sample synthesized at 150 °C. | ||
Both the 27Al and 29Si MAS NMR spectra suggest the presence of Si–O–T (T = Si, Al) environments different from those commonly present in Al-MCM-41 obtained by conventional routes, which resemble those existing in faujasite. However, no evidence of the presence of tiny, submicrometer-sized crystals in the samples has been found by TEM or Ar adsorption. Moreover, no bands corresponding to zeolite framework vibrations are observed in the FTIR spectrum (ESI†). Therefore, the structural and textural properties of the material can be best explained by assuming that the T–O–T connectivity of the tetrahedral sites located in the pore wall has been substantially altered in comparison with that of Al-MCM-41. However, no evidence on the presence of SBU precursors of Y zeolite has been found in these materials.
Chemical analysis (TXRF) of the sample synthesized at 150 °C and calcined indicates that Na/Al = 0.1 and Si/Al = 2.7 (a Si/Al ratio of 3.0 was obtained by XEDS in the electron microscope).
Catalytic activity of the calcined samples in m-xylene conversion indicates that a deep modification of the T–O–T connectivity exists in the vicinity of the protonic acid sites. Table 1 collects the activity and selectivity of the calcined samples synthesized at 150 °C (ZPM-150) and 175 °C (ZPM-175), as well as those of two aluminium-rich Al-MCM-41 materials and an USY catalyst (CBV 720, a0 = 2.428 nm). The activity of the samples derived from colloidal faujasite precursors is nearly two orders of magnitude higher than that of Al-MCM-41. Indeed, the activity of the new materials approaches that of USY. The para/ortho-xylene (P/O) ratio is 1, the expected value for a large pore catalyst with no shape-selectivity effects.11 It is remarkable that the isomerization/disproportionation (I/D) ratio is close to 10, whereas I/D ratios for faujasite zeolites are always below 2.12 The overall catalytic behaviour indicates, in our opinion, the absence of zeolite nanocrystals in the solid.
| Sample | Si/Al ratioc | R 0 d | P/O ratioe | I/D ratioe |
|---|---|---|---|---|
| a m-Xylene:N2 = 1∶4. b a 0= 2.428 nm. c Bulk. d Reaction rate extrapolated at zero time (mol g−1 h−1). e At ca. 5% m-xylene conversion (see text). | ||||
| USYb | 15 | 1.4 | 0.9 | 1 |
| Al-MCM-41-3 | 5.0 | 7.4 × 10−3 | 1.0 | 9 |
| Al-MCM-41-15 | 5.9 | 7.5 × 10−3 | 0.9 | 19 |
| ZPM-150 | 2.7 | 3.2 × 10−1 | 0.9 | 9 |
| ZPM-175 | 2.6 | 1.8 × 10−1 | 1.1 | 12 |
Taking into account that the I/D ratio decreases strongly as the acid strength increases, our results suggest that this new synthesis route gives rise to porous structured aluminosilicates of enhanced acidity, their acid strength being nevertheless lower than that of USY. These materials are suitable for many catalytic processes that do not require strong acid sites, such as isomerization or hydroisomerization, mild hydrocracking and a number of hydroprocessing reactions, as well as cracking of bulky feedstocks.
We acknowledge the assistance of Dr Teresa Blasco in collecting the solid state NMR spectra, and the financial support of CICYT (project MAT2000-1167-C02-02).
Notes and references
- A. Karlsson, M. Stöcker and R. Schmidt, Microporous Mesoporous Mater., 1999, 27, 181 CrossRef CAS; M. J. Verhoef, P. J. Kooyman, J. C. van der Waal, M. S. Rigutto, J. A. Peters and H. van Bekkum, Chem. Mater., 2001, 13, 683 CrossRef CAS; D. T. On and S. Kaliaguine, Angew. Chem., Int. Ed., 2001, 40, 3248 CrossRef CAS.
- Y. Liu, W. Zhang and T. J. Pinnavaia, Angew. Chem., Int. Ed., 2001, 40, 1255 CrossRef CAS; Y. Liu, W. Zhang and T. J. Pinnavaia, Stud. Surf. Sci. Catal., 2001, 135, 1337 Search PubMed; Z. Zhang, Y. Han, L. Zhu, R. Wang, Y. Yu, S. Qiu, D. Zhao and F-S. Xiao, Angew. Chem., Int. Ed., 2001, 40, 1258 CrossRef CAS.
- Y. Liu, W. Zhang and T. J. Pinnavaia, J. Am. Chem. Soc., 2000, 122, 8791 CrossRef CAS.
- Spain Pat. Appl., ES200201821.
- B. J. Schoeman, J. Sterte and J. E. Otterstedt, Zeolites, 1994, 14, 110 CrossRef CAS.
- I. Díaz, J. Pérez-Pariente and E. Sastre, Stud. Surf. Sci. Catal., 1999, 125, 53 Search PubMed.
- R. B. Borade and A. Clearfield, Catal. Lett., 1995, 31, 267 Search PubMed.
- G. J. Ray and A. Samoson, Zeolites, 1993, 13, 410 CrossRef CAS.
- M. T. Janicke, C. C. Landry, S. C. Christiansen, S. Birtalan, G. D. Stucky and B. F. Chmelka, Chem. Mater., 1999, 11, 1342 CrossRef CAS.
- S. Biz and M. G. White, J. Phys. Chem. B, 1999, 103, 8432 CrossRef CAS.
- J. A. Martens, J. Pérez-Pariente, E. Sastre, A. Corma and P. A. Jacobs, Appl. Catal., 1988, 45, 85 CrossRef CAS.
- A. Corma, V. Fornés, J. Pérez-Pariente, E. Sastre, J. A. Martens and P. A. Jacobs, ACS Symp. Ser., 1988, 368, 555 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: N2 isotherm of sample ZPM-150. FTIR spectra of samples ZPM-150, Al-MCM-41-15 and USY. See http://www.rsc.org/suppdata/cc/b2/b209983g/ |
| This journal is © The Royal Society of Chemistry 2003 |
