Definitive identification of cysteine and glutathione complexes of bismuth by mass spectrometry: assessing the biochemical fate of bismuth pharmaceutical agents
Neil
Burford
*a,
Melanie D.
Eelman
a,
David E.
Mahony
b and
Michael
Morash
b
aDepartment of Chemistry, Dalhousie University, Halifax, Nova Scotia B3H 4J3, Canada. E-mail: neil.burford@dal.ca
bDepartment of Immunology and Microbiology, Dalhousie University, Halifax, Nova Scotia B3H 4J3, Canada
First published on 3rd December 2002
Abstract
Solutions containing BiCl3, bismuth subsalicylate or Bi(NO3)3 with L-cysteine, DL-homocysteine, D-methionine or glutathione have been examined by electrospray mass spectrometry. Prominent peaks are assigned to bismuth complexes of these biomolecules and provide insight towards understanding the bioactivity of bismuth compounds.
Despite a long history of medicinal applications for many bismuth compounds, the mechanisms of bioactivity are not understood.1–4 The thiophilicity of bismuth5 has prompted speculation that sulfur-based biomolecules represent the primary target for pharmaceuticals such as ‘colloidal bismuth subcitrate’ (CBS) and ‘bismuth subsalicylate’ (BSS). Bismuth complexes of such ligands have been characterised by 13C NMR spectroscopy6–11 and X-ray absorption spectroscopy,12 but structural assignments and formulations are speculative, as the compounds are difficult to isolate. Electrospray mass spectral (ESI-MS) data provide definitive assignments for fragments and molecular species arising from solutions of such systems,13 as demonstrated below (summarised in Table 1) for solutions containing BiCl3, BSS or Bi(NO3)3 with L-cysteine (CYS), DL-homocysteine (HCYS) and glutathione (GSH).†
| m/z | Assignment | Relative Int. (%) | MS/MS m/z | Assignment |
|---|---|---|---|---|
| CYS | ||||
| 449 | 1 | 45 | 328 | 2 |
| 328 | 2 | 100 | 241 | BiS |
| 209 | Bi | |||
| 241 | BiS | 40 | ||
| 209 | Bi | 10 | ||
| HCYS | ||||
| 818 | 3 | 5 | 342 | 5 |
| 477 | 4 | 100 | 342 | 5 |
| 209 | Bi | |||
| 342 | 5 | 55 | 241 | BiS |
| 209 | Bi | |||
| 209 | Bi | 35 | ||
| GSH | ||||
| 821 | 6 | 30 | 514 | 4 |
| 514 | 7 | 100 | 385 | 5 |
| 385 | 8 | 35 | 209 | Bi |
| 308 | HGSH | 90 |
Irrespective of reaction stoichiometry (1∶1, 1∶3, 1∶5), reaction mixtures involving CYS show prominent peaks at m/z 449 and 328 (Fig. 1), that are assigned to the monocationic bismuth complexes 1 and 2,‡ respectively. Formation of the bisthiolate complex (1) is consistent with previously reported solid state structures for aminothiolate derivatives.14 Cation 2 can be described as a bismuth complex containing the dianionic conjugate base of CYS and is modelled on the crystallographically characterised dimethyl derivative, penicillaminatobismuth chloride.15 In addition, tandem mass spectra of m/z 445 (1) gave 328 (2), and m/z 328 (2) gave m/z 241 (BiS+) and 209 (Bi+).
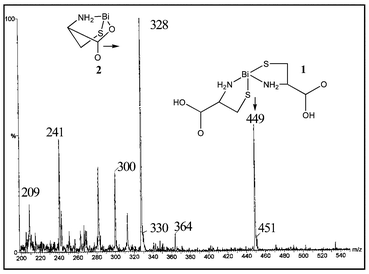 | ||
| Fig. 1 A representative ESI-MS of a solution containing BSS and CYS. | ||
ESI-MS of reaction mixtures containing BiCl3 or BSS and HCYS in aqueous solution, shown in Fig. 2, contain peaks at m/z 818, 477 and 342, which correspond to the tethered dibismuth cation (3) as well as the bis- (4) and mono-thiolate (5) complexes, respectively. ESI-MS/MS of ion 3 (m/z 818) gives a fragment at m/z 342 (5), m/z 445 (4) fragments to give m/z 342 (5) and m/z 209 (Bi+), and m/z 342 (5) gives 241 (BiS+) and m/z 209 (Bi+).
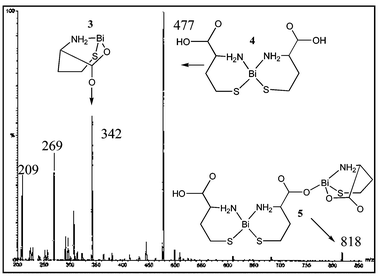 | ||
| Fig. 2 A representative ESI-MS of a solution containing BSS and HCYS. | ||
ESI-MS of solutions containing BSS or Bi(NO3)3 with GSH (Fig. 3) reveal analogous complexes to those observed for the bismuth–cysteine mixtures, in contrast to observations for the antimony–glutathione system.16 These observations were also independent of stoichiometry (1∶1 or 1∶2). Peaks at m/z 821, 514 and 385 are assigned to 6, 7, and 8, respectively. ESI-MS/MS of cation 6 gives fragments at m/z 514 (7), which releases glutamic acid to give m/z 385 (8). The same cations are observed using a MALDI source.§
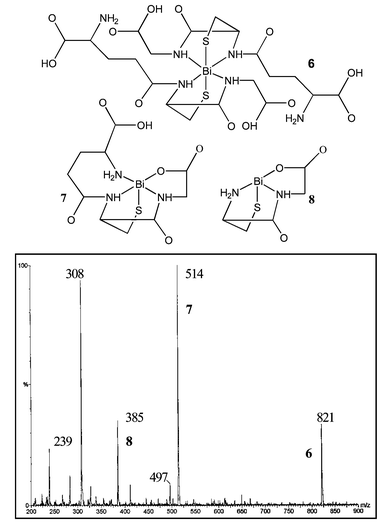 | ||
| Fig. 3 A representative ESI-MS of a solution containing Bi(NO3)3 and GSH. | ||
Bismuth–methionine complexes could not be observed in solutions of BiCl3, Bi(NO3)3 or BSS with D-methionine, despite previous reports of such complexes,17 suggesting the importance of the thiolate anchor in the formation of bismuth complexes.
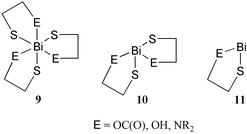
ESI-MS of reaction mixtures provide definitive identification data for bismuth complexes involving CYS, HCYS and GSH. The proposed formulations are important in the quest to understand the bioactivity of bismuth compounds,15,18,19 and are consistent with the established series of tris- 9, bis- 10 and mono- 11 thiolate complexes that have been previously isolated and comprehensively characterised.14,20,21
The interactions of bismuth with weakly donating functional groups of the hetero-bifunctional ligands in these complexes are made possible by the thermal and hydrolytic stability of the sulfur–bismuth bond which serves as an anchor for the ligand.5,21 In this context, the observations described above provide support for the thiolation of bismuth as the primary biochemical fate of bismuth pharmaceuticals.
We thank the Natural Sciences and Engineering Research Council of Canada, MDS Sciex, the Killam Foundation, the Canada Research Chairs Program, the Canada Foundation for Innovation and the Nova Scotia Research and Innovation Trust Fund for funding, and the Dalhousie Mass Spectrometry Laboratory and MDS Sciex for use of instrumentation.
Notes and references
- P. J. Sadler, H. Li and H. Sun, Coord. Chem. Rev., 1999, 185–186, 689–709 CrossRef CAS.
- P. J. Sadler and Z. Guo, Pure Appl. Chem., 1998, 70, 863–871 CAS.
- G. G. Briand and N. Burford, Chem. Rev., 1999, 99, 2601–2657 CrossRef CAS.
- J. Reglinski, Chemistry of Arsenic, Antimony and Bismuth, ed. N. C. Norman, Blackie Academic & Professional, London, 1998, pp. 403–440 Search PubMed.
- G. G. Briand and N. Burford, Adv. Inorg. Chem., 2000, 50, 285–357 Search PubMed.
- G. Alonzo, N. Bertazzi and M. Consiglio, Inorg. Chim. Acta, 1984, 85, L35–L37 CrossRef CAS.
- H. Sun, H. Li, A. B. Mason, R. C. Woodworth and P. J. Sadler, J. Biol. Chem., 2001, 276, 8829–8835 CrossRef CAS.
- L. Zhang, K. Y. Szeto, W. B. Wong, T. T. Loh, P. J. Sadler and H. Sun, Biochemistry, 2001, 40, 13281–13287 CrossRef CAS.
- A. Napoli, Ann. Chim., 1982, 72, 575–583 CAS.
- H. Sun, H. Li and P. J. Sadler, J. Inorg. Biochem., 1995, 59, 190 CrossRef.
- P. J. Sadler, H. Sun and H. Li, Chem. Eur. J., 1996, 2, 701–708 CAS.
- H. Sun, H. Li, I. Harvey and P. J. Sadler, J. Biol. Chem., 1999, 274, 29094–29101 CrossRef CAS.
- N. Burford, M. D. Eelman and T. S. Cameron, Chem. Commun., 2002, 1402–1403 RSC.
- G. G. Briand, N. Burford, T. S. Cameron and W. Kwiatkowski, J. Am. Chem. Soc., 1998, 120, 11374–11379 CrossRef.
- W. A. Herrmann, E. Herdtweck and L. Pajdla, Chem. Ber., 1993, 126, 895–898 CAS.
- H. Sun, S. C. Yan and W. S. Cheng, Eur. J. Biochem., 2000, 267, 5450–5457 CrossRef CAS.
- C. A. McAuliffe, J. V. Quagliano and L. M. Vallarino, Inorg. Chem., 1966, 5, 1996–2003 CrossRef CAS.
- P. V. Radheshwar, R. Dev and G. H. Cady, J. Inorg. Nucl. Chem., 1972, 34, 3913–3915 CrossRef CAS.
- S. P. Summers, K. A. Abboud, S. R. Farrah and G. J. Palenik, Inorg. Chem., 1994, 33, 88–92 CrossRef CAS.
- G. G Briand, N. Burford and T. S. Cameron, Chem. Commun., 2000, 13–14 RSC.
- L. Agocs, G. G. Briand, N. Burford, T. S. Cameron, W. Kwiatkowski and K. N. Robertson, Inorg. Chem., 1997, 36, 2855–2860 CrossRef CAS.
Footnotes |
| † Samples were injected directly at a flow rate of 5 μl min−1 into the electrospray source of a VG Micromass Quattro triple quadrupole mass spectrometer or an API 3000 PE Sciex triple quadrupole mass spectrometer, with a source temperature of 385 K and skimmer cone voltage of 50 V. MS/MS spectra were obtained using argon as a collision gas with a collision energy equal to 50 eV. The argon pressure was sufficient to reduce the intensity of the main beam by 1%. BSS (2.93 mmol) and CYS (13.5 mmol) [or BiCl3 (3.52 mmol) and CYS (10.6 mmol) or CYS (3.49 mmol)] in distilled water (100 mL) stirred overnight at RT and suction filtered. BSS (3.88 mmol) and HCYS (3.91 mmol) or HCYS (6.05 mmol) [or BiCl3 (3.52 mmol) and HCYS (10.6 mmol)] in distilled water (150 mL) stirred for 6 h and suction filtered. Bi(NO3)3 (3.63 mmol) and GSH (3.71 mmol) or GSH (7.52 mmol) [or BSS (3.20 mmol) and GSH (3.11 mmol)] in distilled water (150 mL) stirred for 2 h and suction filtered. BSS (3.01 mmol) and D-methionine (2.99 mmol) [or BiCl3 (3.21 mmol) or Bi(NO3)3 (2.86 mmol) in distilled water, stirred for 3 h and suction filtered. |
| ‡ Molecular drawings represent monocationic species and illustrate connectivity only; drawings of these complexes aimed at describing bonding features (e.g. Lewis) are not meaningful or are misleading. |
| § MALDI MS was performed on a Micromass MALDI LR mass spectrometer using 0.01 g of α-cyano-4-hydroxycinnamic acid in a 50/50 mixture of acetonitrile and ethanol as the matrix. |
| This journal is © The Royal Society of Chemistry 2003 |