Employing the simple monosilylcopper reagent, Li[PhMe2SiCuI], in 1,4-addition reactions†
Jesse
Dambacher
and
Mikael
Bergdahl
*
Department of Chemistry, San Diego State University, San Diego, CA 92182-1030, USA. E-mail: bergdahl@sciences.sdsu.edu
First published on 3rd December 2002
Abstract
Conjugate addition reactions using the simple Li[PhMe2SiCuI] reagent to a variety of α,β-unsaturated carbonyl compounds is described; dimethyl sulfide from the purification of CuI plays a key role for very high yields as well as high stereoselectivities in the formation of β-silyl carbonyl compounds.
Carbon–silicon bonds have fundamental roles in organic synthesis, for example as control moieties for stereochemistry in chemical reactions,1 or as precursors to introduce a hydroxy function.2 Among the silyl substituted variants available, the dimethylphenylsilyl group is very useful because it can be removed under oxidative conditions (Tamao–Fleming)3,2b to make alcohols via retention of stereochemistry.2a Hence, establishing the carbon–silicon bond configuration in the β-position to a carbonyl group serves as a complementary way of making enantiomerically pure aldol fragments. The most frequent way of introducing a PhMe2Si group to a β-carbonyl carbon is via the conjugate addition of a silylcuprate reagent to an α,β-unsaturated carbonyl compound.4b,5
Much attention has been focused on the silylcyanocuprates, depicted as (PhMe2Si)2Cu(CN)Li2,6a or Li[(PhMe2Si)2Cu]LiCN,6b as PhMe2Si donors over the last two decades,4a yet it is rather surprising that little consideration has been paid to the simpler Li[PhMe2SiCuI]7 reagent in conjugate addition reactions. We now report that the Li[PhMe2SiCuI] reagent works extraordinarily well, which counters previous indications.8 The monosilylcopper reagent is more useful than expected for 1,4-type additions (Scheme 1).
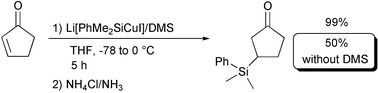 | ||
| Scheme 1 | ||
We have obtained high yields of products in the conjugate addition process employing the simple monosilylcopper reagent even without external additives, such as tributylphosphine,8b HMPA,8a or dialkylzinc.9 In THF at −78 °C, Li[PhMe2SiCuI] was prepared from 1 equiv of LiSiMe2Ph and 1 equiv10 of CuI, purified via its dimethyl sulfide complex.11 The corresponding α,β-unsaturated substrate was added at −78 °C, and the reaction temperature was increased toward 0 °C over a period of 5–8 h depending on the reactivity of the starting material. Representative results are shown in Table 1.12
| Entry | Substrate | Producta | Yield (%)b | |
|---|---|---|---|---|
| a Characterized by NMR (1H and 13C), IR and MS. b Isolated and purified. c Reactions were conducted using a CuI·0.75DMS complex. d Ratio of diastereomers. e 99.999% reagent purity CuI (Aldrich). f 0.75 equivalents of DMS vs. CuI. | ||||
| 1 |
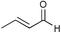
|
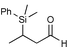
|
82c | |
| 2 |
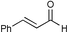
|
|
71c | |
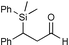
| 3 |
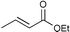
|
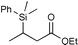
|
95c | |
| 4 |
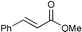
|
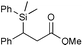
|
91c | |
| 5 |
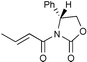
|
|
99c | |
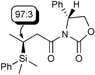
| Ratiod | ||||
| 6 |
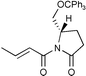
|
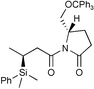
|
92c | 96∶4 |
| 7 | 63e | 1∶1 (−DMS) | ||
| 8 | 76e | 85∶15 (+DMS)f | ||
| 9 |
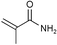
|
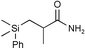
|
60c | |
The Li[PhMe2SiCuI] reagent seems mild enough to work in the presence of fairly sensitive substrates, and slow enough to prompt diastereoselective additions. Our results obtained with the simple monosilylcopper reagent are superior to those from other known silylcopper classes on the same substrates,13 not only with respect to chemical yield, but also levels of stereoselectivity. High chemical yields (71–99%) using α,β-unsaturated ketones, aldehydes, and esters, suggests that there is little, if any, oligomerization. We found the monosilylcopper reagent obtained from CuI/DMS undergoes efficient conjugate additions to moderately unreactive α,β-unsaturated substrates with imides as well as esters, where excellent isolated yields (>90%) were obtained (entries 5 and 6). Remarkably, Li[PhMe2SiCuI] also underwent a conjugate addition with an α,β-unsaturated amide in 60% yield (entry 9), showing that it is possible to conduct a conjugate addition in the presence of fairly acidic protons. A significant advantage in terms of cost and reducing chemical waste is that preparing Li[PhMe2SiCuI] requires only one equivalent of PhMe2SiLi, whereas other commonly used silyl copper reagents, e.g. Li[(PhMe2Si)2Cu]LiCN, require two equivalents of PhMe2SiLi per CuCN.
The Li[PhMe2SiCuI] reagent was scrutinized in asymmetric conjugate additions to some optically active α,β-unsaturated substrates, (entries 5–8). In entry 5,14 not only is the chemical yield almost quantitative but also de is 94% for the conjugate addition of the monosilylcopper reagent. This result is somewhat surprising since tributylphosphine was reported ‘imperative’ for the Li[PhMe2SiCuI] reagent to undergo a conjugate addition to an imide, even at 0 °C.8b Likewise, the Li[(PhMe2Si)2Cu]LiCN reagent typically gives 50% de to the same substrate. Moreover, Koga’s glutamic acid based auxiliary15,16 is a very efficient chiral director for asymmetric conjugate addition of Li[PhMe2SiCuI] (entry 6). As a final point, the present Li[PhMe2SiCuI] system is more attractive than the introduced silylzincate, PhMe2SiZnEt2Li,9b because yields are comparable, levels of diastereomeric excess are higher, and the monosilylcopper reagent is easier to make.
As for the copper source, CuI purified via its dimethyl sulfide (DMS) complex is crucial for the Li[PhMe2SiCuI] reagent to undergo a smooth 1,4-addition reaction. Applying one equivalent of the Aldrich 99.999% grade CuI, versus one equivalent of LiSiMe2Ph in THF, without the presence of DMS, provided only 50% of the β-silylated product (Scheme 1). The presence of DMS has dramatic effects on the rate, stability and selectivity of BuCu/LiI, as reported by Bertz.17 DMS remaining from the purification process of CuI seems likely a component responsible for the higher yield, possibly due to a more soluble silylcopper reagent. Additionally, the presence of DMS plays a fundamental role in favoring the formation of one major diastereomer in the conjugate addition. Thus, employing Koga’s 2-pyrrolidinone (entry 7) and the Li[PhMe2SiCuI] reagent made from the 99.999% grade CuI, in the absence of DMS, not only lowered the yield from 92% to 63%, it also reduced the diastereomeric ratio of the conjugate addition product from 92% to 0% de! When DMS was combined with the 99.999% grade CuI, the yield and the diasteromeric ratio of the β-silylated product increased significantly (entry 8).
The CuI·DMS complex initially formed in the purification process of CuI has been reported to be unstable and loses DMS rapidly to form the more stable CuI·0.75DMS stoichiometry.11 At that point, the DMS concentration remains essentially constant.18 A four month old sample of CuI·0.75DMS taken from a light-protected bottle, gave identical yields and stereoselectivities in the conjugate addition reactions as a freshly prepared sample of CuI·0.75DMS.
This paper shows that excellent results in diastereoselective conjugate additions can be obtained employing the monosilylcopper reagent, Li[PhMe2SiCuI]. It is suggested that the CuI should be purified via its DMS complex prior to making the monosilylcopper reagent. We have also demonstrated that external additives such as tributylphosphine, HMPA, or dialkylzinc are unnecessary, even though only one equivalent, not two, of silyl lithium reagent is used. Employing the simple Li[PhMe2SiCuI] reagent obtained from CuI, could be an attractive alternative to the use of CuCN, which (although it provides catalytic activity)5a is used in conjunction with stoichiometric quantities of the R2Zn reagents. In summary, the Li[PhMe2SiCuI] system is expected to be a very useful reagent, for the chemo- and diastereoselective formation of silicon–carbon bonds in organic synthesis.
This work was funded by RSCA and FGIA SDSU Foundation. The authors wish to thank Dr Le Roy Lafferty and Dr Sam Somanathan for technical assistance.
Notes and references
- (a) E. W. Colvin, Silicon in Organic Synthesis, Butterworths, London, 1981 Search PubMed; (b) W. P. Webber, Silicon Reagents in Organic Synthesis, Springer, Berlin, 1983 Search PubMed; (c) E. W. Colvin, Silicon Reagents in Organic Synthesis, Academic Press, Orlando, FL, 1988 Search PubMed; (d) M. A. Brook, Silicon in Organic, Organometallic and Polymer Chemistry, Wiley, New York, 2000. Search PubMed; (e) I. Fleming, A. Barbero and D. Walter, Chem. Rev., 1997, 97, 2063–2192 CrossRef CAS.
- (a) I. Fleming, R. Henning and H. Plaut, J. Chem. Soc., Chem. Commun., 1984, 29–31 RSC; (b) I. Fleming and P. E. J. Sanderson, Tetrahedron Lett., 1987, 28, 4229–4232 CrossRef CAS; (c) I. Fleming, R. Henning, D. C. Parker, H. E. Plaut and P. E. J. Sanderson, J. Chem. Soc., Perkin Trans. 1, 1995, 317–337 RSC.
- (a) K. Tamao, T. Tanaka, T. Nakajima, R. Sumiya, H. Arai and Y. Ito, Tetrahedron Lett., 1986, 27, 3377–3380 CrossRef CAS; (b) K. Tamao, A. Kawachi and Y. Ito, J. Am. Chem. Soc., 1992, 114, 3989–3990 CrossRef CAS.
- (a) D. J. Ager, I. Fleming and S. K. Patel, J. Chem. Soc., Perkin Trans. 1, 1981, 2520–2526 RSC; (b) I. Fleming, in Organocopper Reagents—A Practical Approach; ed. R. J. K Taylor, Oxford University Press, Oxford, 1994, pp. 267–292, and references therein Search PubMed; (c) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon Press, Oxford, 1992 Search PubMed.
- For some developments of 1,4-additions of silyl groups, see (a) B. H. Lipshutz, J. A, Sclafani and T. Takanami, J. Am. Chem. Soc., 1998, 120, 4021–4022 CrossRef CAS; (b) H. Ito, T. Ishizuka, J. Tateiwa, M. Sonoda and A. Hosomi, J. Am. Chem. Soc., 1998, 120, 11196–11197 CrossRef CAS; (c) T. W. Lee and E. J. Corey, Org. Lett., 2001, 3, 3337–3339 CrossRef CAS.
- (a) B. H. Lipshutz and B. James, J. Org. Chem., 1994, 59, 7585–7587 CrossRef CAS; (b) S. H. Bertz, G. Miao and M. Eriksson, Chem. Commun., 1996, 815–816 RSC.
- As there is no evidence for the Cu–I bond, the monosilylcopper reagent could also be depicted as PhMe2SiCu/LiI.
- (a) K. Takaki, T. Maeda and M. Ishikawa, J. Org. Chem., 1989, 54, 58–62 CrossRef CAS; (b) C. Palomo, J. M. Aizpurua, M. Iturburu and R. Urchegui, J. Org. Chem., 1994, 59, 240–244 CrossRef CAS.
- (a) W. Tückmantel, K. Oshima and H. Nozaki, Chem. Ber., 1986, 119, 1581–1593 Search PubMed; (b) R. A. N. C. Crump, I. Fleming and C. J. Urch, J. Chem. Soc., Perkin Trans. 1, 1994, 701–706 RSC; (c) B. L. MacLean, K. A. Hennigar, K. W. Kells and R. D. Singer, Tetrahedron Lett., 1997, 38, 7313–7316 CrossRef CAS.
- Molar equivalents calculated for pure (CuI)4(Me2S)3, so as to avoid formation of the corresponding homocuprate, (PhMe2Si)2CuLi/LiI. For a discussion on trialkylsilylcuprate formation, see S. Sharma and A. C. Oehlschlager, J. Org. Chem., 1989, 54, 5383–5387 Search PubMed.
- For a discussion on the stability of CuI/DMS complex, see E. Eriksson, A. Hjelmencrantz, M. Nilsson and T. Olsson, Tetrahedron, 1995, 51, 12631–12644 Search PubMed . See also M. Eriksson, T. Iliefski, M. Nilsson and T. Olsson, J. Org. Chem., 1997, 62, 182–187 CrossRef.
- Typically, reactions were conducted in 1 mmol scale (∼60 mM, THF) using 1.4–2.0 equivalents of Li[PhMe2SiCuI].
- Li[PhMe2SiCuI]/DMS gives low yields of addition to β,β-disubstituted enones. Additives, such as TMSI, MgBr2(OEt2), BF3OEt2, or Yb(OTf)3, did not influence the yields of the desired products.
- E. Nicolás, K. C. Russel and V. J. Hruby, J. Org. Chem., 1993, 58, 766–770 CrossRef CAS . For conjugate additions of monoorganocuprate reagents, Li[RCuI], to this substrate, see P. Pollock, J. Dambacher, R. Anness and M. Bergdahl, Tetrahedron Lett., 2002, 43, 3693–3697 Search PubMed.
- K. Tomioka, T. Suenaga and K. Koga, Tetrahedron Lett., 1986, 27, 369–372 CrossRef CAS.
- I. Fleming and N. D. Kindon, J. Chem. Soc., Chem. Commun., 1987, 1177–1179 RSC.
- S. H. Bertz and G. Dabbagh, Tetrahedron, 1989, 45, 425–434 CrossRef CAS . See also E. J. Corey and R. L. Carney, J. Am. Chem. Soc., 1971, 93, 7318–7319 Search PubMed; R. D. Clark and C. H. Heathcock, Tetrahedron Lett., 1974, 1713–1715 CrossRef CAS.
- Confirmed by us using elemental analysis.
Footnote |
| † Electronic supplementary information (ESI) available: Typical experimental procedure, 1H-NMR, 13C-NMR, MS and IR spectral data for all β-silyl carbonyl compounds (PDF). See http://www.rsc.org/suppdata/cc/b2/b210792a/ |
| This journal is © The Royal Society of Chemistry 2003 |
