Self-assembly of a molecular figure-of-eight strip†
Kai P.
Strotmeyer
a,
Igor O.
Fritsky
b,
Hans
Pritzkow
a and
Roland
Krämer
*a
aAnorganisch-Chemisches Institut der Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: roland.kraemer@urz.uni-heidelberg.de; Fax: (+49) 6221 548599; Tel: (+49) 6221 548438
bDepartment of Chemistry, National Taras Shevchenko University, 01033 Kiev, Ukraine. E-mail: ifritsky@ukr.ne; Fax: (+380) 44 2278982; Tel: (+380) 44 2393393
First published on 26th November 2003
Abstract
A hexanuclear copper(II) complex with a figure-of-eight strip topology is formed by metal-directed self-assembly of tritopic ligand L, bis-bidentate glycine hydroxamic acid and Cu(II) ions in a 2:2:6 ratio.
A figure-of-eight strip is produced by twisting a ribbon through 360° and joining its two ends. Here we describe the unprecedented formation of a molecule with this exotic topology1 by self-assembly. On the molecular level, figure-of-eight topologies have been observed, for instance, for covalent macrocyclic ring systems (such as expanded porphyrins)2 and their metal complexes.3 Such compounds are highly dynamic due to rotation around single bonds, while a strip-like topology does not allow interconversion of the helical enantiomers without bond disruption.
Directed self-assembly of “programmed” organic ligands with transition metal ions is a powerful method for the construction of nanometer-sized, complex supramolecular architectures;4 examples include polyhedra, helicates, grids, bowls, capsules or polymeric networks. The tritopic ligand L (Scheme 1) was prepared in a four step reaction from ethyl picolinate and 1,3-diaminopropane. The coordination chemistry of a related ligand has been investigated in detail in a different context:5 in a 1:1 copper complex the metal ion is bound to the tetradentate site and the complex has a helical structure with tetrahedrally distorted N4Cu coordination plane.
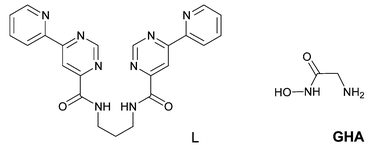 | ||
| Scheme 1 | ||
When L is mixed with 3 equiv. CuCl2 and 1 equiv. glycine hydroxamic acid (GHA) (Scheme 2) in the presence of base in MeOH/water, green crystals of [(L-2H)2Cu6(GHA-2H)2(OH)0.5(ClO4)1.5(Cl,ClO4)(H2O)4.32](ClO4)·10.64 H2O (1) formed on addition of NaClO4 and were investigated by X-ray crystallography.‡
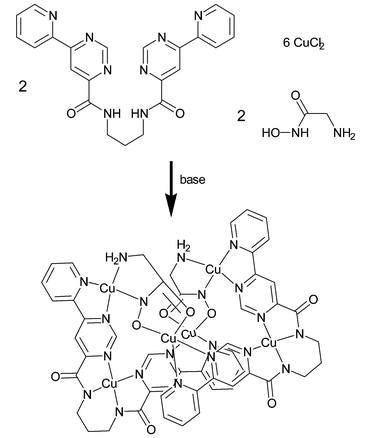 | ||
| Scheme 2 Formation of the complex cation of 1 by self-assembly from L, GHA and CuCl2. Axial Cu-ligands are omitted for clarity. | ||
The unit cell contains a hexanuclear complex cation (Scheme 2) composed of two doubly-deprotonated L (L-2H), two GHA dianions and 6 Cu2+ ions as well as disordered coligands OH−, H2O, ClO4−, Cl−. One Cu2+ ion is tightly bound to the tetradentate site of L by two deprotonated amide nitrogen atoms and two pyrimidine N-Atoms. As a consequence of pyrimidine C2-H repulsion, the LCu moiety is not planar. Its intrinsic helicity is crucial for the assembly of the figure-of-eight topology, and the helicity of the two subunits is essentially the same.
The remaining two Cu ions of an LCu3 unit coordinate to the bidentate pyridyl-pyrimidinyl sites. All Cu ions form 4 short in-plane bonds (distances 1.89–2.02 Å), and the coordination spheres are completed to square-pyramidal or octahedral by weak axial interactions with highly disordered coligands. The two LCu3 fragments are linked by two GHA dianions which coordinate to the cis-oriented vacant sites of the peripheral Cu ions and bridge the Cu ions in a bis-bidentate O,O- and N,N chelating fashion. Previous examples for the use of α-amino hydroxamic acids in metal-directed self-assembly reactions are the synthesis of “metallacrowns”, e.g. from GHA and Cu2+ in combination with a heterometal ion,6 and of helicates.7
Fig. 1 illustrates the figure-of-eight strip topology of the hexanuclear complex cation which has a length of about 1.5 nm and a width of about 1.0 nm. The racemic crystal contains both helical enantiomers in a 1:1 ratio. Atom connectivities in 1 do not allow the interconversion of the helical isomers by rotation around single bonds.
![Top: Illustration of the figure-of-eight strip topology of the complex cation of 1
(weakly bound axial ligands omitted for clarity). Bottom: Structure of the [(L2-2H)2Cu6(GHA-2H)2(OH)0.5(ClO4)1.5(Cl,ClO4)(H2O)4.32] cation of 1 including axial ligands.](/image/article/2004/CC/b309215a/b309215a-f1.gif) | ||
| Fig. 1 Top: Illustration of the figure-of-eight strip topology of the complex cation of 1 (weakly bound axial ligands omitted for clarity). Bottom: Structure of the [(L2-2H)2Cu6(GHA-2H)2(OH)0.5(ClO4)1.5(Cl,ClO4)(H2O)4.32] cation of 1 including axial ligands. | ||
A crystalline sample of 1 is homogeneous: five crystals selected from two independent synthetic crops had identical unit cell parameters, and the presence of only one solid phase was confirmed by powder diffraction. Attempts to identify the intact complex cation of 1 in methanolic solution by electrospray ionisation MS failed, only signals of Na+ adducts of the mononuclear complex (L-2H)Cu or of its oligomers were found, with loss of the peripheral Cu ions under electrospray MS conditions.
Formation of 1 appears to be both enthalpy and entropy driven. In a complex (L-2H)Cu3(GHA-2H), the hydroxamate ligand could not bridge the two peripheral Cu ions in a bis(bidentate) fashion so that vacant sites at Cu and non-coordinated donor atoms at GHA remain, corresponding to a loss of enthalpy relative to 1. Additionally, 1 appears to stabilised by intramolecular stacking interactions. The crossing point of the molecular loop lies approximately between Cu(2a) and Cu(2b), so that the basal planes of these Cu centres are stacked together with a metal–metal distance of 3.612(1) Å. Further, interactions of pyridyl and pyrimidyl heterocycles belonging to different molecules of L are observed, with shortest interatomic contacts of about 3.39 A. Higher oligomer combinations of (L-2H)Cu3 and GHA in 1:1 ratio would be disfavoured by entropy.
In conclusion, the first example of formation of a molecular figure-of-eight strip by self-assembly is reported. Combination of the helical LCu3 building block with bis- or tris-bidentate ligands other than GHA might yield assemblies of different topology and higher complexity.
This work was supported by the Deutsche Forschungsgemenischaft (Gerhard Hess-Programm) and the Fonds der Chemischen Industrie.
Notes and references
- Surprisingly, a single crystalline µm-sized figure-of-eight strip of NbSe3 could be generated under specific crystal growth conditions: S. Tanda, T. Tsumeta, Y. Okajima, K. Inagaki, K. Yamaya and N. Hatakenaka, Nature, 2002, 417, 397–398 Search PubMed.
- E. Vogel, M. Broering, J. Fink, D. Rosen, H. Schmickler, J. Lex, K. W. K. Chan, Y.-D. Wu and D. Plattner, Angew. Chem. Int. Ed. Engl., 1995, 34, 2511–2514 CAS; N. Sprutta and L. Latos-Grazynski, Chem. Eur. J., 2001, 7, 5099–5112 CrossRef CAS; J. L. Sessler, D. Seidel, A. Gebauer, V. Lynch and K. A. Abboud, J. Heterocycl. Chem., 2001, 38, 1419–1424 CAS; S. Brooker, G. S. Dunbar and T. Weyhermüller, Supramol. Chem., 2001, 13, 601–612 CAS.
- P. Comba, A. Kuhner and A. Peters, J. Chem. Soc., Dalton Trans., 1999, 509–516 RSC; P. Comba, A. Fath, T. W. Hambley, A. Kuehner, D. T. Richens and A. Vielfort, Inorg. Chem., 1998, 37, 4389–4401 CrossRef CAS; X. Li, J. Illigen, M. Nieger, S. Michel and C. Schalley, Chem. Eur. J., 2003, 9, 1332–1347 CrossRef CAS.
- Recent reviews: K. L. Thompson, Coord. Chem. Rev., 2002, 233/234, 193–206 Search PubMed; S. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972–983 CrossRef; M. D. Ward, J. A. McCleverty and J. C. Jeffery, Coord. Chem. Rev., 2001, 222, 251–272 CrossRef CAS; D. W. Johnson and K. N. Raymond, Supramol. Chem., 2001, 13, 639–659 CrossRef CAS; M. Albrecht, Chem. Rev., 2001, 101, 3457–3497 CrossRef CAS; M. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa and K. Biradha, Chem. Commun., 2001, 509–518 CrossRef CAS; G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3529 RSC.
- I. O. Fritsky, R. Ott and R. Krämer, Angew. Chem. Int. Ed., 2000, 39, 3255–3258 CrossRef CAS; I. O. Fritsky, R. Ott, H. Pritzkow and R. Krämer, Chem. Eur. J., 2001, 7, 1221–1231 CrossRef CAS.
- A. J. Stemmler, J. W. Kampf, M. L. Kirk, B. H. Atasi and V. L. Pecoraro, Inorg.Chem., 1999, 38, 2807–2817 CrossRef CAS; A. D. Cutland, R. G. Malkani, J. W. Kampf and V. L. Pecoraro, Angew. Chem. Int. Ed., 2000, 39, 2689–2691 CrossRef CAS; J. J. Bodwin, A. D. Cutland, R. G. Malkani and V. L. Pecoraro, Coord. Chem. Rev., 2001, 216–217, 489–512 CrossRef CAS.
- J. A. Johnson, J. W. Kampf and V. L. Pecoraro, Angew. Chem. Int. Ed., 2003, 42, 546–549 CrossRef.
Footnotes |
| † Dedicated to Prof. Dr. Bernt Krebs on the occasion of his 65th birthday. |
| ‡ Crystal data for 1: [(L-2H)2Cu6(GHA-2H)2(OH)0.5(ClO4)1.5(Cl,ClO4)(H2O)4.32](ClO4)·10.64 H2O (1), M = 2008.9, monoclinic, C2/c, a = 45.980(5), b = 12.546(1), c = 26.557(3) Å, β = 110.935(2), V = 14308(11) Å3, T = 106(2) K, Z = 8, ρcalc = 1. 865 g cm−3, 12585 independent reflections, R1 = 0.0577 (I > 2σ(I), wR2 = 0.1646 (all reflections)). CCDC 217223. See http://www.rsc.org/suppdata/cc/b3/b309215a/ for crystallographic data in .cif or other electronic format.Crystal structure determination data were collected with a Bruker AXS Area Detector (MoKα radiation, ω-scans) at low temperature. Structures were refined by least-squares methods based on F2 with all reflections (Bruker AXS SHELXTL 5.1). |
| This journal is © The Royal Society of Chemistry 2004 |
