Gas-phase reactions for selective detection of the explosives TNT and RDX
Eduardo C.
Meurer
a,
Hao
Chen
b,
Leah
Riter
b,
Ismael
Cotte-Rodriguez
b,
Marcos N.
Eberlin
*a and
R. Graham
Cooks
*b
aInstitute of Chemistry, State University of Campinas, UNICAMP, SP, Brazil. E-mail: eberlin@iqm.unicamp.br; Fax: +55-19-3788-3073
bDepartment of Chemistry, 560 Oval Drive, Purdue Univeisty, West Lafayette, IN, USA 47907. E-mail: cooks@purdue.edu; Fax: + 1-765-494-9421
First published on 20th November 2003
Abstract
Highly selective gas-phase reactions with ethyl vinyl ether (EVE) of major electron (EI) and chemical ionization (CI) fragment ions of the explosives TNT and RDX have been uncovered. The fragment ion of m/z 210 from TNT undergoes [4+ + 2] cycloaddition with EVE to form an oxo-iminium ion of m/z 282, which dissociates by acetaldehyde loss after a [1,5-H] shift to form a quinolynium ion of m/z 238. The fragment ion of m/z 149 from RDX reacts with EVE by a formal vinylation reaction, that is, the elusive cyclic adduct loses ethanol to yield a nitro-iminium ion of m/z 175, which reacts further with EVE to form a second cyclic product ion of m/z 247. Calculations and MS/MS experiments support the proposed structures. These highly characteristic reactions of diagnostic EI and CI fragment ions improve selectivity for TNT and RDX detection.
Explosives detection is a topic of increasing interest.1 For such an important task, several analytical methods have been developed, including gas chromatography either with electron capture or nitrogen phosphorus detectors, mass spectrometry2 (MS) and high performance liquid chromatography with ultraviolet or MS detection.3 Improved performance, including greater selectivity (to minimize false positives) and sensitivity, remains highly desirable and tandem mass spectrometry4 is an obvious candidate. While collision-induced dissociation is very selective, highly complex matrices call for additional selectivity. This might be provided by ion/molecule reactions of mass-selected ions with appropriate neutral reagents. Previous experiments on trinitrotoluene (TNT) suggest that such gas-phase reactions can provide an excellent MS method for rapid, sensitive, and highly selective chemical analysis.5
Gas phase cycloadditions are highly selective for compound class and geometry, and are therefore prime candidates for diagnostic reactions. We have systematically studied a variety of such reactions by tandem MS.6 For instance, N-alkylpiperidienes form N-alkyl-2-azabutadienyl cations upon 70 eV electron ionization (EI) and these ions undergo structurally diagnostic [4+ + 2] cycloadditions with vinyl ethers followed by prompt alcohol loss.6d Herein we report that highly selective cycloaddition or cyclization reactions (Scheme 1) occur for the TNT fragment ion of m/z 210 (1) and the hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) fragment ion of m/z 149 (2) with ethyl vinyl ether (EVE). Proposed product ion structures are corroborated via MS/MS experiments and AM1 calculations. The EVE cycloaddition of 1, an iminium ion, is similar to that of N-alkyl-2-azabutadienyl cations,6a,b whereas the EVE cyclization reaction of 2 (a formal vinylation reaction) is unprecedented.
![Reaction mechanisms of vinylation for the ion 2 of m/z 149 and [4+
+ 2] cycloaddition for the ion 1 of m/z 210.](/image/article/2004/CC/b309962h/b309962h-s1.gif) | ||
| Scheme 1 Reaction mechanisms of vinylation for the ion 2 of m/z 149 and [4+ + 2] cycloaddition for the ion 1 of m/z 210. | ||
Interestingly, we found 1 and 2 also to be formed as the major product ions via chemical ionization (CI) of TNT and RDX with 1,2-dichloroethane through H2O and CH2NNO2 loss, respectively.7 This increases the sensitivity of the analysis making 1 and 2 excellent candidates for both EI and CI diagnostic ions in TNT and RDX detection. Ions 1 and 2 were individually mass-selected and reacted with EVE and Fig. 1 shows the resulting mass spectra. The ion of m/z 282 (Fig. 1a) is likely the expected regioselective [4+ + 2] EVE cycloadduct6c of 1, which loses acetaldehyde after a [1,5-H] shift to form the second product ion of m/z 238. For 2, the intact [4+ + 2] EVE adduct of m/z 221 is not observed likely owing to prompt ethanol loss that forms the ion of m/z 175 (Fig. 1b). Then, cyclization of m/z 175 with a second EVE molecule forms m/z 247. Competitive reactions in both cases form protonated EVE of m/z 73 and ethylated EVE of m/z 101,6b whereas CID of 1 forms m/z 193, m/z 164, and that of 2 forms m/z 85 and m/z 75 (not shown).
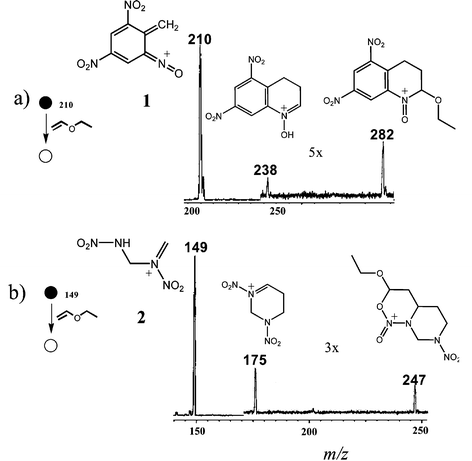 | ||
| Fig. 1 Product ion mass spectra showing the ion/molecule reactions of EVE with a) ion 1 of m/z 210 and b) ion 2 of m/z 149. | ||
Potential energy surface diagrams for the proposed reactions of 1 and 2 with EVE as elaborated from semi-empirical AM1 calculations8 show that both reactions are highly exothermic (Fig. 2). Because they are abundant in 70 eV EI and CI spectra, 1 and 2 are important target ions for TNT and RDX, respectively; hence, their characteristic reactions with EVE herein described function as a highly diagnostic method for TNT and RDX detection.
![Potential energy surface diagram for [4+
+ 2] cycloaddition and subsequent aldehyde loss from 1 and EVE (right pathway) and cyclization with fast ethanol loss and a second EVE addition from 2 and EVE (left pathway). The energies of the full geometry optimized species in kcal mol−1 are: 1 ion (258.38), EVE (−31.33), adduct1 (1
− EVE)
(192.59), adduct2 (1
− EVE − acetaldehyde)
(185.81), product (quinolynium)
(226.07), acetaldehyde (−41.56), 2 ion (265.39), adduct3 (2
− EVE)
(197.38), adduct4 (2
− EVE − ethanol)
(264.12), adduct5 [(2
− EVE − ethanol)
− EVE]
(192.33) and ethanol (−62.66).](/image/article/2004/CC/b309962h/b309962h-f2.gif) | ||
| Fig. 2 Potential energy surface diagram for [4+ + 2] cycloaddition and subsequent aldehyde loss from 1 and EVE (right pathway) and cyclization with fast ethanol loss and a second EVE addition from 2 and EVE (left pathway). The energies of the full geometry optimized species in kcal mol−1 are: 1 ion (258.38), EVE (−31.33), adduct1 (1 − EVE) (192.59), adduct2 (1 − EVE − acetaldehyde) (185.81), product (quinolynium) (226.07), acetaldehyde (−41.56), 2 ion (265.39), adduct3 (2 − EVE) (197.38), adduct4 (2 − EVE − ethanol) (264.12), adduct5 [(2 − EVE − ethanol) − EVE] (192.33) and ethanol (−62.66). | ||
This work was supported by NSF (grant CHE327190) and ONR (N00014-02-0834) and the IDHM Program, Center for Sensing Science and Technology (Purdue University) and the NSWC (Crane, Indiana). E.M. and M.N.E. acknowledge funding from FAPESP – Brazil and L.R. from Sandia National Lab.
Notes and references
- J. Yinon, Anal. Chem., 2003, 75, 99A CAS.
- (a) M. E. Sigman, C. Ma and R. H. Ilgner, Anal. Chem., 2001, 73, 792 CrossRef CAS; (b) A. B. Fialkov, A. Gordin and A. Amirav, J. Chromatogr., A, 2003, 991, 217 CrossRef CAS.
- (a) R. Batlle, H. Carlsson, E. Holmgren, A. Colmsjo and C. Crescenzi, J. Chromatogr., A, 2002, 963, 73 CrossRef CAS; (b) S. D. Harvey and T. R. W. Clauss, J. Chromatogr., A, 1996, 753, 81 CrossRef CAS; (c) P. M. Gates, E. T. Furlong, T. F. Dorsey and M. R. Burkhardt, Trends Anal. Chem., 1996, 15, 319 CrossRef CAS; (d) J. Hawari, A. Halasz, C. Groom, S. Deschamps, L. Paquet, C. Beaulieu and A. Corriveau, Environm. Sci. Technol., 2002, 36, 5117 Search PubMed.
- K. L. Busch, G. L. Glish and S. A. McLuckey, MS/MS: Techniques and Applications of Tandem Mass Spectrometry, VCH Publishers, Inc., New York, 1988 Search PubMed.
- (a) K. C. Crellin, M. Widmer and J. L. Beauchamp, Anal. Chem., 1997, 69, 1092 CrossRef CAS; (b) L. S. Riter, D. F. Fraley and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2000, 11, 33 CrossRef CAS.
- (a) R. Augusti, F. C. Gozzo, L. A. B. Moraes, R. Sparrapan and M. N. Eberlin, J. Org. Chem., 1998, 63, 4889 CrossRef CAS; (b) E. C. Meurer and M. N. Eberlin, Int. J. Mass Spectrom., 2001, 210, 469 CrossRef; (c) M. N. Eberlin and R. G. Cooks, J. Am. Chem. Soc., 1993, 115, 9226 CrossRef CAS; (d) L. A. B. Moraes and M. N. Eberlin, J. Mass Spectrom., 2002, 37, 162 CrossRef CAS; (e) E. C. Meurer, R. Sparrapan and M. N. Eberlin, J. Mass Spectrom., 2003, 38, 1075 CrossRef.
- H. Chen, X. Zheng, P. Yang and R. G. Cooks, manuscript in preparation.
- M. J. S. Dewar, E. G. Zoebisch, E. F. Healy and J. P. P. Stewart, J. Am. Chem. Soc., 1985, 107, 3902 CrossRef.
| This journal is © The Royal Society of Chemistry 2004 |
