Practical synthesis of new and highly efficient ligands for the Suzuki reaction of aryl chlorides
Alexander
Zapf
a,
Ralf
Jackstell
a,
Franck
Rataboul
a,
Thomas
Riermeier
b,
Axel
Monsees
b,
Christa
Fuhrmann
a,
Nadim
Shaikh
a,
Uwe
Dingerdissen
b and
Matthias
Beller
*a
aLeibniz-Institut für Organische Katalyse an der Universität Rostock e.V., Buchbinderstr. 5-6, 18055 Rostock, Germany
bDegussa AG, Creavis Technologies & Innovation, Project House Catalysis, Industriepark Höchst, G830, 65926 Frankfurt am Main, Germany
First published on 28th November 2003
Abstract
A practical synthesis of a novel class of phosphine ligands, phosphino substituted N-aryl pyrroles (PAP ligands), has been developed. These ligands are applied in the palladium-catalyzed coupling of a variety of aryl and heteroaryl chlorides with phenylboronic acid showing exceedingly high turnover numbers at mild reaction temperatures and even at room temperature.
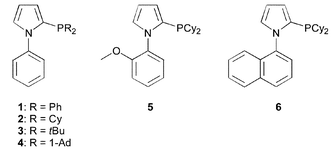
Biaryls constitute important building blocks for the synthesis of biologically active substances, e.g. pharmaceuticals and herbicides.1 Currently, the most versatile method for the synthesis of substituted biaryls is the cross-coupling reaction of aryl halides and arylboronic acids (Suzuki reaction).2 Suzuki cross-coupling reactions of aryl halides have been extensively studied in organic synthesis and the methodology has been significantly enlarged in the last decade. Recently, even catalyst-free Suzuki reactions of aryl bromides and iodides were developed using microwave technology.3 However, less reactive chloroarenes, which are easily accessible with a variety of substituents and are often the most economically interesting substrates do not react under these conditions. Notable catalyst developments for the use of aryl chlorides4 have been reported by Bedford,5 Buchwald,6 Fu,7 Herrmann,8 Nolan,9 us10 and others.11 Our main interest is concerned with the practical application of palladium-catalyzed coupling reactions.12 In this respect the most active ligands for Suzuki reactions, which are composed of sterically demanding basic phosphines, still have significant drawbacks such as the high sensitivity towards oxygen and/or the price (availability) of the ligand. Therefore the development of more efficient ligands, which lead to highly active catalyst systems and can easily be prepared and modified on a larger scale, is still an important topic in this area. Here, we describe for the first time the synthesis and application of new 2-phosphino-1-arylpyrrole ligands 1–6 (PAP ligands, Fig. 1), which fulfil these criteria.
Faigl and co-workers have shown that N-aryl pyrroles can be deprotonated selectively at the α-position to the nitrogen atom.13 We thought that reaction of these carbanions with chlorophosphines should give access to novel ligands, which would be sterically demanding because of their biaryl backbone and electron rich, if suitable chlorophosphines were chosen. Indeed lithiation of different N-aryl pyrroles, with n-BuLi/TMEDA and subsequent reaction with chlorodiphenylphosphine, chlorodicyclohexylphosphine, di-1-adamantylchlorophosphine and di-tert-butylchlorophosphine gave the PAP ligands 1–6 directly in one step. Initially, ligands 1–3 were tested in the coupling of 2-chlorotoluene with phenylboronic acid under various reaction conditions.
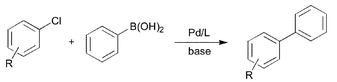 | ||
| Scheme 1 Suzuki coupling of aryl chlorides with phenylboronic acid. | ||
The screening of PAP–Ph (1), PAP–Cy (2) and PAP-t-Bu (3) in the presence of 0.1 mol% Pd(OAc)2 or 0.05 mol% Pd2dba3 (L/Pd = 2/1, toluene) and different bases demonstrates the importance of basic substituents on the phosphorus atom: almost no yield (and conversion) is observed if 1 is applied as ligand. On the other hand, with 2 and 3 as ligands high yields (> 90%) have been observed. Advantageously, best yields have been obtained using potassium phosphate or cheap potassium carbonate as base.
The general usefulness of our new catalyst systems is shown in Table 1. 15 different aryl and heteroaryl chlorides gave the corresponding biaryls in good to excellent yield (74 to > 99%) at low catalyst concentration (0.05 mol% Pd or below). While a number of substrates have been tested in the presence of all synthesized ligands, most often PAP–t-Bu (3) gave the best results. For example para-substituted chloroarenes are coupled efficiently in the presence of only 0.01 mol% palladium and 0.02 mol% 3. Utilizing 3 electron rich aryl chlorides (entries 5 and 7; 80–99%) are coupled as smoothly as electron deficient ones (entries 1 and 2; >99%). Consistently, chlorobenzene yields biphenyl in 96% yield under the same conditions (entry 3). Noteworthy are the mild reaction conditions (60 °C) under which the different coupling reactions proceed. Even at room temperature good yields of biaryl products can be obtained in the presence of 0.1 mol% Pd. To the best of our knowledge the obtained turnover number of 800 for the coupling of a non-activated aryl chloride (4-chlorotoluene; entry 4) at room temperature is the highest ever reported. When the reaction temperature is increased to 100 °C the catalyst concentration can be reduced to 0.005 mol% without any decrease in yield, resulting in a TON of almost 20,000 (entry 6). However, when the catalyst concentration is further lowered (0.001 mol% of catalyst) the activity breaks down and product yields are generally below 40%.
| Entry | Aryl chloride | Ligand | Cat. conc. [mol%] | Temp. [°C] | Yield [%] | TON |
|---|---|---|---|---|---|---|
| 1 |
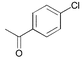
|
3 | 0.01 | 60 | > 99 | 10,000 |
| 2 |
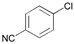
|
3 | 0.01 | 60 | > 99 | 10,000 |
| 3 |
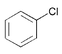
|
3 | 0.01 | 60 | 96 | 9600 |
|
4
5 6 |
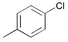
|
3
3 3 |
0.1
0.01 0.005 |
r.t.
60 100 |
80
99 98 |
800
9900 19,600 |
| 7 |
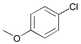
|
3 | 0.01 | 60 | 80 | 8000 |
| 8 |
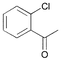
|
3 | 0.05 | 60 | 80 | 1600 |
| 9 |
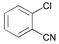
|
3 | 0.05 | 60 | >99 | 10,000 |
|
10
11 |
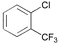
|
3
3 |
0.05
0.01 |
60
100 |
90
91 |
1800
9100 |
| 12 |
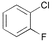
|
3 | 0.01 | 60 | 97 | 9700 |
| 13 |

|
3 | 0.01 | 60 | 97 | 9700 |
|
14
15 16 17 18 |
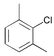
|
2
3 4 5 6 |
0.05
0.05 0.05 0.05 0.05 |
60
60 60 60 60 |
72
16 15 55 35 |
1440
320 300 1100 700 |
| 19 |
|
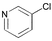
3 | 0.01 | 60 | 99 | 9900 |
| 20 |
|
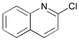
3 | 0.05 | 60 | 87 | 1740 |
| 21 |
|
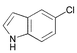
3 | 0.05 | 100 | 97 | 1940 |
| 22 |
|
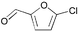
3 | 0.05 | 100 | 99 | 1980 |
In the case of ortho-substituted aryl chlorides the catalytic efficiency is strongly dependent on the bulk of the substituent. Relatively large or rigid groups result in the need of a higher catalyst concentration (0.05 mol%) or higher reaction temperatures (100 °C) in order to obtain good yields even if the electronic nature of the substituent leads to a formal activation of the substrate (acetyl, cyano, trifluoromethyl groups; entries 8–11). Ortho-substituents that do not interfere with the metal complex, e.g. fluoro or methoxy groups (entries 12 and 13), have no detrimental effect on the reaction outcome. However, when two ortho-methyl substituents are present in the chloroarene, dicyclohexylphosphino substituted ligands, especially 2, result in more efficient catalysts than the more bulky tert-butyl or adamantyl derivatives (entries 14–18).
Heteroaryl chlorides can also be arylated efficiently using the new PAP ligands. For example, 3-chloropyridine yields 99% of 3-phenylpyridine in the presence of 0.01 mol% catalyst (entry 19). 2-Chloroquinoline requires some more catalyst (0.05 mol%) to be coupled well (87%, entry 20), because of a supposed dimerization of the oxidative addition product via coordination of the nitrogen atom to a second palladium center. For the coupling of 5-chloroindole and 5-chlorofurfural, respectively, the reaction temperature needs to be increased to 100 °C. Then, almost quantitative yields (>97%) are obtained (entries 21 and 22).
In summary, we have shown that monodentate 2-phosphino-1-arylpyrrole ligands 1–6 (PAP ligands) can be prepared directly from N-aryl pyrroles. Hence, ligand synthesis of the PAP family is comparably easy and the shown PAP ligands will be commercially available on the 100 g-scale.14 The new ligand family allows highly efficient coupling reactions of electron rich as well as electron poor aryl chlorides with phenylboronic acid under mild conditions. In general, excellent yields are obtained at very low catalyst concentrations.
Financial support by the state of Mecklenburg-Western Pomerania, the BMBF, and Degussa AG are gratefully acknowledged. We thank OMG for gifts of palladium compounds. The authors thank Mr. M. Hoff (Degussa AG) for his excellent technical assistance.
References and notes
-
(a) N. Yasuda, J. Organomet. Chem., 2002, 653, 279 CrossRef CAS
; (b) H.-U. Blaser, A. F. Indolese and A. Schnyder, Curr. Sci., 2000, 78, 1336 Search PubMed
.
-
(a) A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS
; (b) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS
.
-
(a) N. E. Leadbeater and M. Marco, J. Org. Chem., 2003, 68, 5660 CrossRef CAS
; (b) N. E. Leadbeater and M. Marco, Angew. Chem., 2003, 115, 1445 CrossRef
.
- A. F. Littke and G. C. Fu, Angew. Chem., 2002, 114, 4350 CrossRef
.
-
(a) R. B. Bedford, Chem. Commun., 2003, 1787 RSC
; (b) R. B. Bedford, C. S. J. Cazin, S. J. Coles, T. Gelbrich, P. N. Horton, M. B. Hursthouse and M. E. Light, Organometallics, 2003, 22, 2810 CrossRef CAS
; (c) R. B. Bedford, M. E. Blake, C. P. Craig and D. Holder, Chem. Commun., 2003, 466 RSC
; (d) R. B. Bedford, C. S. J. Cazin, S. J. Coles, T. Gelbrich, P. N. Horton, M. B. Hursthouse and M. E. Light, Organometallics, 2003, 22, 987 CrossRef CAS
; (e) R. B. Bedford, C. S. J. Cazin and S. L. Hazelwood, Angew. Chem., 2002, 114, 4294 CrossRef
.
-
(a) J. P. Wolfe and S. L. Buchwald, Angew. Chem., 1999, 111, 2570 CrossRef
; (b) J. P. Wolfe, R. A. Singer, B. H. Yang and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 9550 CrossRef CAS
.
-
(a) S.-Y. Liu, M. J. Choi and G. C. Fu, Chem. Commun., 2001, 2408 RSC
; (b) A. F. Littke, C. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020 CrossRef CAS
; (c) A. F. Littke and G. C. Fu, Angew. Chem., 1998, 110, 3586 CrossRef
.
-
(a) C. W. K. Gstöttmayr, V. P. W. Böhm, E. Herdtweck, M. Grosche and W. A. Herrmann, Angew. Chem., 2002, 114, 1421 CrossRef
; (b) V. P. W. Böhm, C. W. K. Gstöttmayr, T. Weskamp and W. A. Herrmann, J. Organomet. Chem., 2000, 595, 186 CrossRef CAS
.
-
(a) M. S. Viciu, R. F. Germaneau, O. Navarro-Fernandez, E. D. Stevens and S. P. Nolan, Organometallics, 2002, 21, 5470 CrossRef CAS
; (b) G. A. Grasa, M. S. Viciu, J. Huang, C. Zhang, M. L. Trudell and S. P. Nolan, Organometallics, 2002, 21, 2866 CrossRef CAS
; (c) A. C. Hillier, G. A. Grasa, M. S. Viciu, H. M. Lee, C. Yang and S. P. Nolan, J. Organomet. Chem., 2002, 653, 69 CrossRef CAS
; (d) C. Zhang, J. Huang, M. L. Trudell and S. P. Nolan, J. Org. Chem., 1999, 64, 3804 CrossRef CAS
.
-
(a) A. Zapf and M. Beller, Chem. Eur. J., 2000, 6, 1830 CrossRef CAS
; (b) A. Zapf, A. Ehrentraut and M. Beller, Angew. Chem., 2000, 112, 4315 CrossRef
; (c) M. Gómez Andreu, A. Zapf and M. Beller, Chem. Commun., 2000, 2475 RSC
; (d) M. Beller, H. Fischer, W. A. Herrmann, K. Öfele and C. Broßmer, Angew. Chem., 1995, 107, 1992 CrossRef
.
-
(a) R. A. Singer, S. Caron, R. E. McDermott, P. Arpin and N. M. Do, Synthesis, 2003, 1727 CAS
; (b) Q.-S. Hu, Y. Lu, Z.-Y. Tang and H.-B. Yu, J. Am. Chem. Soc., 2003, 125, 2856 CrossRef CAS
; (c) J. P. Stambuli, R. Kuwano and J. F. Hartwig, Angew. Chem., 2002, 114, 4940 CrossRef
; (d) A. Schnyder, A. F. Indolese, M. Studer and H.-U. Blaser, Angew. Chem., 2002, 114, 3820 CrossRef
; (e) G. Y. Li, J. Org. Chem., 2002, 67, 3643 CrossRef CAS
; (f) L. Botella and C. Najera, Angew. Chem., 2002, 114, 187 CrossRef
; (g) A. Fürstner and A. Leitner, Synlett, 2001, 290 CAS
; (h) G. Y. Li, Angew. Chem., 2001, 113, 1561 CrossRef
; (i) C. Zhang and M. L. Trudell, Tetrahedron Lett., 2000, 41, 595 CrossRef CAS
; (j) X. Bei, T. Crevier, A. S. Guram, B. Jandeleit, T. S. Powers, H. W. Turner, T. Uno and W. H. Weinberg, Tetrahedron Lett., 1999, 40, 3855 CrossRef CAS
.
-
(a) M. Beller and A. Zapf, Top. Catal., 2002, 19, 101 CrossRef
; (b) M. Beller, A. Zapf and W. Mägerlein, Chem. Eng. Technol., 2001, 24, 575 CrossRef CAS
; (c) J. G. de Vries, Can. J. Chem., 2001, 79, 1086 CrossRef CAS
.
-
(a) F. Faigl, K. Fogassy, E. Szucs, K. Kovács, G. M. Keseru, V. Harmat, Z. Böcskei and L. Toke, Tetrahedron, 1999, 55, 7881 CrossRef CAS
; (b) F. Faigl, K. Fogassy, A. Thurner and L. Toke, Tetrahedron, 1997, 53, 4883 CrossRef CAS
; (c) F. Faigl and M. Schlosser, Tetrahedron, 1993, 49, 10271 CrossRef CAS
.
- CataCXium® P, Degussa Homogeneous Catalysts, Degussa AG, Düsseldorf.
| This journal is © The Royal Society of Chemistry 2004 |