Renewable hydrogen by aqueous-phase reforming of glucose†
Rupali R.
Davda
and
James A.
Dumesic
*
University of Wisconsin, Madison, 1415 Engineering Drive, Madison, Wisconsin 53706, USA. E-mail: dumesic@engr.wisc.edu; Fax: 1 608 262 5434; Tel: 1 608 262 1095
First published on 20th November 2003
Abstract
Hydrogen can be produced from aqueous solutions containing 10 wt% glucose with high selectivities through the combined use of a hydrogenation reactor for conversion of glucose to sorbitol, followed by a reforming reactor for conversion of sorbitol to H2 and CO2 and then a gas–liquid separator for the removal of high-pressure H2-rich reformate gas, ready for use in a fuel cell.
Biomass is a promising source for the sustainable production of hydrogen in an age of diminishing fossil fuel reserves; however, conversion of biomass to hydrogen remains a challenge, since processes such as enzymatic decomposition of sugars, steam-reforming of bio-oils and gasification suffer from low hydrogen production rates and/or complex processing requirements.1
We have recently reported2 that hydrogen can be generated by catalytic reforming of oxygenated hydrocarbons in liquid water at temperatures near 500 K. Of particular interest in this class of reactants is glucose (C6O6H12), because this sugar makes up the major energy reserves in plants and animals. While the selectivity for hydrogen production is insensitive to the liquid-phase concentration of sugar-alcohols such as sorbitol (C6O6H14), the hydrogen selectivity from reforming of glucose decreases as the liquid concentration increases from 1 to 10 wt% because of undesired hydrogen-consuming side reactions that occur in the liquid phase.3 This decrease in selectivity is an important limitation, because processing dilute aqueous solutions involves the processing of excessive amounts of water.
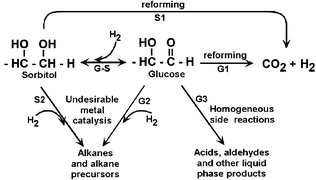
Fig. 1 depicts various reaction pathways that take place during aqueous-phase reforming of glucose and sorbitol. Production of H2 and CO2 from glucose (G) and sorbitol (S) takes place on metal catalysts such as Pt or NiSn alloys (pathways G1 and S1) via cleavage of C–C bonds followed by water-gas shift processes.2 Undesired alkanes are formed by cleavage of C–O bonds on the metal catalyst and by dehydration processes on acidic catalyst supports (pathways G2, S2).4 In the case of glucose, undesired reactions can also take place in the aqueous phase to form organic acids, aldehydes and carbonaceous deposits (pathway G3).3 These undesirable homogeneous decomposition reactions are first order in glucose concentration, whereas the desirable reforming reactions on the catalyst surface are fractional order;5 therefore, high concentrations of glucose lead to low hydrogen selectivities. Another reaction that links the aforementioned pathways is the hydrogenation of glucose to sorbitol (pathway G–S), which takes place on metal catalysts with high selectivity at low temperatures (e.g., 400 K) and high H2 pressures.6
The rates of pathways G1, G2, G3 increase more rapidly with temperature than does the rate of pathway G–S. Accordingly, a strategy for improving the hydrogen selectivity from aqueous-phase reforming of glucose is to employ a dual-reactor system involving a low-temperature hydrogenation step followed by a higher-temperature reforming process. To implement this two-step process, it is important to understand how the pressure of H2 depends on the system pressure. Aqueous-phase reforming leads to the production of gas bubbles containing H2, CO2 and alkanes. The pressure in these bubbles is approximately equal to the total pressure of the system, and the partial pressures of the gaseous reaction products and water vapour depend on the composition of the feed stream and the saturation pressure of water at the reactor temperature.7 For this study, the partial pressures of the gas phase products in the reactor were varied by co-feeding a gas stream (N2 or H2) with the liquid feed at the inlet of the reactor. The performance of the reactor in this mode can then be compared to the case where only liquid is fed to the reactor and N2 sweep gas is combined with the effluent stream at the exit of the reactor.
Table 1A and B shows the results of aqueous-phase reforming of 10 wt% sorbitol and glucose solutions at 538 K and 52.4 bar over a Pt/Al2O3 catalyst. All runs were conducted at high conversions of the feed to gas-phase products, from which the reaction selectivities were calculated. We report the hydrogen selectivity, which is defined as the number of moles of H2 in the effluent gas normalized by the number of moles of H2 that would be present if each mole of carbon in the effluent gas had participated in the sorbitol or glucose reforming reaction to give 13/6 or 2 moles of H2, respectively. When H2 is co-fed with the liquid reactant stream to the reactor, the hydrogen selectivity reported is based on the calculated moles of hydrogen produced, using the composition of the carbon-containing products in the effluent gas stream. We also report the alkane selectivity, which is defined as the moles of carbon in the gaseous alkane products normalized by the total moles of carbon in the gaseous effluent stream.
| Experiment | A: Sorbitol reforming | B: Glucose reforming | C: Hydrogenation + reforming | |||||
|---|---|---|---|---|---|---|---|---|
| Gas flow at reactor inlet/outlet | N2 sweep at outlet | N2 fed at inlet | H2 fed at inlet | N2 sweep at outlet | N2 fed at inlet | H2 fed at inlet | Sorbitol + H2 fed at inlet | Glucose + H2 fed at inlet |
| a Catalyst: 3 wt% Pt on nanofibers of γ-Al2O3 (BET area 500 m2 g−1) having a Pt dispersion of 70% (see ESI1). | ||||||||
| Pressure/bar | 52.4 | 52.4 | 52.4 | 52.4 | 52.4 | 52.4 | 54.8 | 54.8 |
| Hydrogenation/reforming WHSV/g(feed) g(cat)−1 h−1 | —/0.0963 | —/0.0224 | 0.091/0.065 | |||||
| % Carbon in gas-phase effluent | 86 | 96 | 91 | 91.2 | 92.4 | 95.8 | 100 | 93 |
| % H2 selectivity | 50 | 62 | 63 | 10.5 | 13.4 | 11.6 | 57.3 | 62.4 |
| % Alkane selectivity | 22 | 20 | 19.1 | 49.5 | 47.5 | 46.8 | 22.7 | 21.3 |
| α = moles H2/mole glucose fed (αmax = 12) | 4.4 | 6.7 | 6.6 | 1.3 | 1.5 | 1.5 | 6.5 | 5.8 |
The highest rates of aqueous-phase reforming were obtained for runs where N2 was combined with the liquid feed at the reactor inlet. Also, since glucose is less reactive than sorbitol for aqueous-phase reforming over Pt/Al2O3,2 lower weight hourly space velocities (WHSV; grams of reactant per gram of catalyst per hour) of glucose were employed to achieve high conversions. Table 1 shows that the hydrogen selectivities observed for aqueous-phase reforming of glucose (∼10–13%) are much lower compared to reforming of sorbitol (∼60%) under similar conditions. Also higher alkane selectivities of 47–50% were observed for reforming of glucose, indicating that aqueous-phase reforming of glucose, even with hydrogen co-fed with the liquid reactant stream at the inlet of the reactor, is not selective for production of H2. The above results indicate that co-feeding hydrogen with liquid reactants into the reforming reactor at 538 K (and 52.4 bar) does not lead to rapid hydrogenation of glucose into sorbitol and its subsequent reforming to give high H2 selectivities. Similarly, sorbitol does not undergo rapid dehydrogenation to glucose under conditions of aqueous-phase reforming where H2 is not co-fed with the liquid reactant stream to the reforming reactor, a desirable result.
Experiments were conducted by co-feeding gaseous H2 with aqueous solutions containing 10 wt% sorbitol or glucose into a dual-reactor system consisting of a hydrogenation reactor at 393 K followed by a reforming reactor. The presence of the hydrogenation reactor did not affect the aqueous-phase reforming of sorbitol. However, when 10 wt% glucose was co-fed with gaseous H2 to the dual-reactor system, high hydrogen selectivity (62.4%) and low alkane selectivity (21.3%) were obtained (see Table 1C), these values being similar to the selectivities obtained from the reforming of sorbitol. These results indicate that glucose was first completely hydrogenated to sorbitol before being sent to the reformer in which the sorbitol was converted with high selectivity to H2 and CO2.
Table 1 compares the net moles of hydrogen produced per mole of glucose (α), where the maximum theoretical value of α is 12. For sorbitol feeds, α is calculated by subtracting 1 from the moles of H2 produced per mole of feed (to account for the extra mole of H2 in sorbitol compared to glucose). For sorbitol reforming, α is ca. 6.6 when N2 or H2 are co-fed with the liquid reactant stream to the reactor. In contrast, α is only 1.5 for the glucose feed. The addition of a hydrogenation reactor upstream of the reformer leads to α equal to nearly 6 for glucose reforming, showing an improvement of 290% in the net production of hydrogen per mole of glucose.
A novel aspect of the approach outlined here for the generation of hydrogen from aqueous solutions of glucose is that we have identified a beneficial synergy formed by operating the hydrogenation reactor, the reformer, and the gas–liquid separator (situated downstream of the reformer) at different temperatures while maintaining the total pressure of the system, Ptot, at a constant value. This situation is depicted schematically in Fig. 2. An aqueous solution of glucose is co-fed with gaseous H2 to the hydrogenation reactor, which is operated at a relatively low temperature, T1, to minimize glucose decomposition reactions in the liquid phase. The partial pressure of hydrogen, PH2, in the reactor is equal to Ptot − PH2O(T1), where PH2O(T1) is the vapor pressure of water at T1. For example, if Ptot is 54.8 bar and T1 is 393 K, then PH2 = 52 bar. This relatively high pressure of hydrogen is favorable for the conversion of glucose to sorbitol. The aqueous solution of sorbitol and gaseous H2 are then fed to the reforming reactor, which is operated at the higher temperature, T2 (e.g., T2 = 538 K), necessary to convert sorbitol to H2 and CO2. Finally, the liquid and gaseous effluents from the reformer are cooled and sent to a separator, which is maintained at a low temperature, T3. The sum of the partial pressures of H2 and CO2 in the separator is equal to Ptot − PH2O(T3). If the separator is at room temperature, then the sum of the H2 and CO2 pressures is essentially equal to Ptot, which is 54.8 bar. This high pressure of the reformate facilitates further removal of CO2 from H2 by pressure-swing adsorption. A fraction of the purified H2 at high pressure can then be recycled to the hydrogenation reactor, and the remaining hydrogen may be directed to a fuel cell for conversion to electrical power.
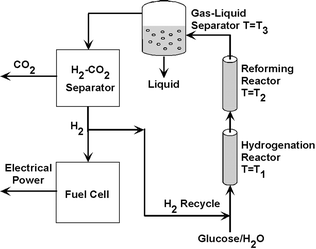 | ||
| Fig. 2 Schematic representation of the recycle loop for generation of hydrogen by aqueous-phase reforming of glucose. | ||
Notes and references
- J. Woodward, M. Orr, K. Cordray and E. Greenbaum, Nature, 2000, 405, 1014 CrossRef CAS; L. Garcia, R. French, S. Czernik and E. Chornet, Appl. Catal., A, 2000, 201, 225 CrossRef CAS.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964 CrossRef CAS; G. W. Huber, J. W. Shabaker and J. A. Dumesic, Science, 2003, 300, 2075 CrossRef CAS; R. R. Davda, J. W. Shabaker, G. W. Huber, R. D. Cortright and J. A. Dumesic, Appl. Catal., B, 2003, 43, 13 CrossRef CAS.
- B. M. Kabyemala, T. Adschiri, R. M. Malaluan and K. Arai, Ind. Eng. Chem. Res., 1999, 38, 2888 CrossRef CAS.
- S. P. Bates and R. A. Van Santen, Adv. Catal., 1998, 42, 1 CAS.
- J. W. Shabaker, R. R. Davda, G. W. Huber, R. D. Cortright and J. A. Dumesic, J. Catal., 2003, 215, 344 CrossRef CAS.
- P. Gallezot, P. J. Cerino, B. Blanc, G. Fleche and P. Fuertes, J. Catal., 1994, 146(1), 93 CrossRef CAS.
- R. R. Davda and J. A. Dumesic, Angew. Chem., Int. Ed., 2003, 42(34), 4068 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: catalyst preparation and characterization; reaction kinetics studies; acknowledgements. See http://www.rsc.org/suppdata/cc/b3/b310152e/ |
| This journal is © The Royal Society of Chemistry 2004 |