Preparation of hybrid thin film modified carbon nanotubes on glassy carbon electrode and its electrocatalysis for oxygen reduction†
Jianying
Qu
,
Yan
Shen
,
Xiaohu
Qu
and
Shaojun
Dong
*
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, China. E-mail: dongsj@ns.ciac.jl.cn; Fax: +86-431-5689711; Tel: +86-431-5262101
First published on 28th November 2003
Abstract
A hybrid thin film containing Pt nanoparticles and [tetrakis(N-methylpyridyl)porphyrinato]cobalt (CoTMPyP) modified multi-walled carbon nanotubes (MWNTs) on a glassy carbon (GC) electrode surface was fabricated. This hybrid film electrode exhibited remarkable electrocatalytic activity for oxygen reduction and high stability with promising applications in fuel cells.
It is well known that the studies of oxygen reduction are very important for the hydrogen–oxygen fuel cell, especially the study of developing electrode catalysts for oxygen reduction with high efficiency and longer lifetime. Among them, the metalloporphyrins were paid more attention because of the extended porphyrin π system. However, most of mononuclear metalloporphyrins serve as catalysts only for the two-electron reduction of oxygen to produce H2O2.1
In recent years, carbon nanotubes (CNTs) have attracted more and more attention from various fields since they were found in 1991.2 The high surface area and hollow geometry, combined with their high electronic conductivity and useful mechanical properties, all exhibit that CNTs have the ability to promote electron transfer reaction when used as electrode materials in electrochemical reaction. Recent research has used CNTs as a component of electrode materials to catalyze biomolecules3,4 and inorganic compounds.5 What is more, CNTs were successfully used in studying direct electrochemistry of proteins.6,7
To date, the only microelectrode constructed with MWNTs and CNTs electrode deposited on metal such as Pd and Ag were reported to catalyze O2 reduction. The catalytic potentials for O2 reduction only shifted to −0.31 V (vs. SCE in 0.01 M H2SO4) and 0.07 V (vs. SCE in 1 M H2SO4), respectively.5
Since the oxygen reduction depends not only on the structure of the catalyst but also on the substrate electrode material upon which it was adsorbed, we prepared the MWNTs/CoTMPyP/Pt hybrid film modified GC electrodes, by combining with all the favorable properties of CNTs, metalloporphyrins and metal nanoparticles. Compared with the results of previous works at GC electrode either modified with only CoTMPyP and Pt nanoparticles,8 or with only MWNTs and metal nanoparticles,5 the present hybrid film modified electrode exhibits more remarkable electrocatalytic activity for the reduction of oxygen through four-electron pathway to yield H2O.
MWNTs (95%) purchased from Shenzhen Nanotech. Port. Co., Ltd (Shenzhen, China) were directly purified by refluxing in 3 M HNO3 for 48 hours, then were filtered with 2.5 µm minipore size membrane with the aid of a pump and thoroughly washed with water to get neutral state, finally dried under vacuum at 60 °C overnight to obtain the purified MWNTs (p-MWNTs) with about 20–40 nm diameter. 20 µL of the black p-MWNTs solution in ethanol (0.1 mg mL−1) was cast on a polished, cleaned and dried GC (d = 3 mm) electrode surface and evaporated the solvent at room temperature to prepare the GC/MWNTs electrode. The GC/MWNTs electrode was immersed in 1mM CoTMPyP(ClO4)5 (synthesized according to a known procedure9) solution (pH3.8 HAc-NaAc) for 1.5 hours, then was taken out and cleaned with water and dried by a high-purity nitrogen stream to give the GC/MWNTs/CoTMPyP electrode. This electrode was put into 1 mM K2PtCl6 aqueous solution for 30 minutes. In this way, a PtCl62− layer was adsorbed on the surface of the GC/MWNTs/CoTMPyP electrode by electrostatic interaction. The above electrode was taken out and washed carefully with water, dried by a nitrogen stream, and then was reduced electrochemically to yield Pt nanoparticles in situ under constant potential at −0.7 V in N2-saturated 0.1 M KCl solution. The one layer of MWNTs/CoTMPyP/Pt hybrid film modified GC electrode was prepared through this method. The multilayer of (MWNTs/CoTMPyP/Pt)n hybrid film modified GC electrodes were constructed in the same way by repeating the above operations alternatively, except that the volume of 0.1 mg mL−1 p-MWNTs solution in each layer was 5 µL.
Fig. 1 (curve a and b) shows the XPS date of the MWNTs/CoTMPyP modified film on GC electrode surface. The peaks of N1s at 399.7 eV, Co2p3/2 at 780.1 eV indicated the cobalt porphyrin was adsorbed on GC electrode modified with MWNTs film. In fact, except for the CoTMPyP on the outer surface of the MWNTs, some CoTMPyP species may be adsorbed into the inner wall of MWNTs due to its porous structure. Fig. 1 (curve c and d) shows the XPS date of the MWNTs/CoTMPyP/PtCl62− film (before reduction) and the MWNTs/CoTMPyP/Pt hybrid film (after reduction), respectively. In curve c, only Pt(IV) 4f7/2 and 4f5/2 peaks at 74.8 and 78.0 eV were presented. After reduction at −0.7 V in N2-saturated 0.1 M KCl solution (as shown in curve d), Pt(0) 4f7/2 peaks at 71.6 and 74.7 occurred, the Pt(IV) 4f5/2 was decreased but not disappeared. These confirmed the formation of Pt(0) and also the incomplete reduction of PtCl62−. Experiments showed that PtCl62− can not be reduced to Pt(0) completely in spite of prolonging of electrochemical reduction for a long time. This phenomenon might be a result of the porous structure and special character of CNTs.
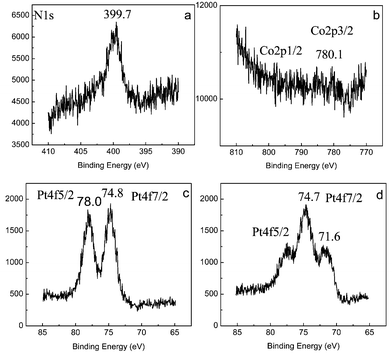 | ||
| Fig. 1 XPS data of MWNTs/CoTMPyP film on GC surface (curve a and b) and MWNTs/CoTMPyP/PtCl62− film in the Pt (4f) region before (curve c) and after (curve d) electrochemical reduction. | ||
The tapping mode AFM images of the MWNTs/CoTMPyP/Pt hybrid film on GC electrode were shown in Fig. 2. Before reduction, only the MWNTs with some bundles can be seen (image A). After electrochemical reduction, the images of Pt nanoparticles were observed clearly on the CNTs with average diameter of 20–40 nm (image B). In fact, we can see that Pt nanoparticles were inserted to the inner wall of the CNTs.
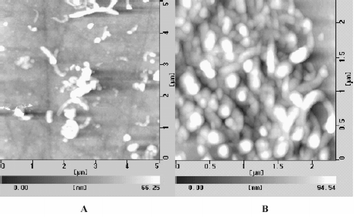 | ||
| Fig. 2 AFM images. (A) MWNTs/CoTMPyP/PtCl62− film assembled on GC electrode (before electrochemically reduction); (B) MWNTs/CoTMPyP/Pt hybrid film (after electrochemically reduction at −0.7 V in N2-saturated 0.1 M KCl solution) | ||
Cyclic voltammetry (CV) measurements were conducted in air-saturated 0.1 M HAc-NaAc (pH 3.8) buffer solution for reduction of oxygen at the ambient temperature in a three-electrode electrochemical cell. The working electrode was GC or modified GC electrodes. A Ag/AgCl (saturated KCl) electrode and a coiled platinum wire were used as the reference and the auxiliary electrode respectively.
In order to compare the electrocatalytic performance at different modified electrodes, we prepared MWNTs/CoTMPyP, CoTMPyP/MWNTs, MWNTs/Pt and MWNTs/CoTMPyP/Pt film modified GC electrodes with the same amount of MWNTs and the same adsorption time of cobalt porphyrins, and the experiments were carried out in the same condition. Fig. 3 shows CV curves of oxygen reduction in 0.1 M HAc-NaAc (pH 3.8) buffer solution at the different modified electrodes. Obviously, the GC/MWNTs/CoTMPyP/Pt electrode is the most efficient one for electrocatalytic reduction of oxygen, a catalytic current occurred at about 0.2 V, than that of other electrodes. Furthermore, the peak current varied linearly with the square root of scan rate (see Fig. S1 of the ESI†), indicating that oxygen reduction at MWNTs/CoTMPyP/Pt hybrid film modified GC electrode was a diffusion controlled process.
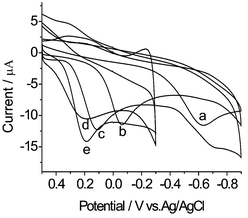 | ||
| Fig. 3 Cyclic voltammograms of O2 reduction at different electrodes. a: blank GC electrode; b: GC/CoTMPyP/MWNTs electrode; c: GC/MWNTs/CoTMPyP electrode; d: GC/MWNTs/Pt electrode; e: GC/MWNTs/CoTMPyP/Pt electrode. Supporting electrolyte: air-saturated 0.1 M HAc-NaAc (pH 3.8) buffer solution. Scan rate: 50 mV s−1. | ||
The one layer of MWNTs/CoTMPyP/Pt hybrid film modified GC electrode was further investigated by rotating disk electrode (RDE) and rotating ring-disk electrode (RRDE) experiments. Through analysis of the above experiments results (see Fig. S2 and Fig. S3 of the ESI†), the n value was evaluated to be about 3.9 and 3.6, respectively, indicating mainly a process of 4e for O2 reduction to water.
This one layer of MWNTs/CoTMPyP/Pt modified GC electrode also was tested to catalyze oxygen reduction in 0.05 M H2SO4, the peak potential shifted more positive to about 0.35 V (not shown). Compared to the catalytic reduction of oxygen at microelectrode with MWNTs (Epc = −0.31 V, vs. SCE, in 0.01 M H2SO4 solution) and MWNTs electrode deposited with metal such as Pd and Ag (Epc = 0.07 V, vs. SCE, in 1 M H2SO4),5 the present electrode exhibited more remarkable electrocatalytic activity for O2 reduction. Although the peak potential of oxygen reduction occurred also to about 0.35 V (vs. Ag/AgCl) in 0.05 M solution at (CoTMPyP/Pt)6 multilayer modified GC electrode,8 in fact, at least 6 layers of (CoTMPyP/Pt) were needed. However, the preparation of one layer of MWNTs/CoTMPyP/Pt hybrid film modified GC electrode for O2 reduction was simple, efficient and more stable.
CV experiments of O2 reduction at multilayer of (MWNT/CoTMPyP/Pt)n hybrid film modified on GC electrodes with different layers (n = 1–7) in 0.1 M acetate buffer solution were performed too. The peak current of O2 reduction was increased and the peak potential shifted to positive direction with increasing of the layer numbers, and the reduction of O2 was almost through 4e at different layers on GC electrodes. The results indicated that the multilayer of (MWNTs/CoTMPyP/Pt)n hybrid film with Pt nanoparticles as outermost layer modified GC electrodes possessed high catalytic activity for oxygen reduction.
The stability of the MWNTs/CoTMPyP/Pt hybrid film modified GC electrode was tested by CV eight times a day successively or was exposed in air for one month, there was negligible change in the shape and height of the catalytic current of O2 reduction, indicating the hybrid film had high electrocatalytic stability for the oxygen reduction. Furthermore, this hybrid thin film was very hard to remove from the GC electrode surface. The only way to remove the film is to polish the electrode mechanically, indicating a high mechanical stability.
In summary, MWNTs/CoTMPyP/Pt hybrid film modified GC electrodes were prepared which exhibited high electrocatalytic activity for oxygen reduction to produce H2O through a four-electron pathway reduction with high stability. It is hoped to develop a new electrode material in fuel cell.
This work was supported by the National Natural Science Foundation of China (No.20275036, No.20211130506) and by the Special Funds for Major State Basic Research of China (No. 2002CB713803). We are grateful to Mr Zhiguo Liu for his help with the AFM experiments.
Notes and references
- E. Song, C. Shi and F. C. Anson, Langmuir, 1998, 14, 4315 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- P. J. Britto, K. S. V. Santhanam and P. M. Ajayan, Bioelectrochem. Bioenerg., 1996, 41, 121 CrossRef CAS.
- J. Wang, M. Li, Z. Shi, N. Li and Z. Gu, Anal. Chem., 2002, 74, 1993 CrossRef CAS.
- P. J. Britto, K. S. V. Santhanam, A. Rubio, J. A. Alonso and P. M. Ajayan, Adv. Mater., 1999, 11, 154 CrossRef CAS.
- W. Huang, S. Taylor, K. Fu, Y. Lin, D. Zhang, T. W. Hanks, A. M. Rao and Y. P. Sun, Nano Lett., 2002, 2, 311 CrossRef CAS.
- J. J. Daris, R. J. Coles, H. Allen and D. Hill, J. Electroanal. Chem., 1997, 440, 279 CrossRef CAS.
- Y. Shen, J. Liu, A. Wu, J. Jiang, L. Bi, B. Liu, Z. Li and S. Dong, Langmuir, 2003, 19, 5397 CrossRef CAS.
- R. F. Pasternack and M. S. Cobb, J. Inorg. Nucl. Chem., 1973, 35, 4327 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figs. S1–S3. See http://www.rsc.org/suppdata/cc/b3/b311038a/ |
| This journal is © The Royal Society of Chemistry 2004 |
