A novel, tandem construction of C–N and C–C bonds: facile and one-pot transformation of the Baylis–Hillman adducts into 2-benzazepines†
Deevi Basavaiah* and Tummanapalli Satyanarayana
School of Chemistry, University of Hyderabad, Hyderabad 500 046, India
First published on 28th November 2003
Abstract
A novel reaction involving tandem construction of C–N and C–C bonds via the simultaneous Ritter and Houben–Hoesch reactions on Baylis–Hillman adducts leading to a convenient, one-pot synthesis of 2-benzazepine derivatives is described. A facile stereoselective transformation of the Baylis–Hillman adducts into (E)- and (Z)-allyl amides is also presented.
The 2-benzazepine moiety is present in many pharmaceutically active naturally occurring molecules such as galanthamine (one of the most effective current drugs for Alzheimer's disease), lycoramine, narwedine, montanine, coccinine, pancracine, brunsvigine, ribasine, communesin A, communesin B, nomofungin, etc.1 and in fact, several synthetic 2-benzazepines have also been found to exhibit hypotensive, analgesic, antiarrhythmic activity and are also useful for treatment of mental disorders and hypoxia.2 Therefore, the development of simple and convenient procedures for the synthesis of 2-benzazepine derivatives continues to be a challenging endeavor in synthetic organic chemistry.1a–d,3 In continuation of our interest in the synthesis of heterocyclic molecules using Baylis–Hillman chemistry,4 we herein describe a novel reaction involving tandem construction of C–N and C–C bonds via the simultaneous Ritter5 and Houben–Hoesch6 reactions on Baylis–Hillman adducts leading to a convenient, one-pot synthesis of 2-benzazepine derivatives.
The Baylis–Hillman reaction is an emerging atom-economical carbon–carbon bond forming reaction providing densely functionalized molecules whose applications in many organic transformation methodologies have been well documented.4,7,8 During our ongoing research program on the synthesis of useful and important heterocyclic molecules,4 we required various 2-benzazepine derivatives. We have envisioned that 2-benzazepine derivatives can in principle be obtained from the Baylis–Hillman adducts via the construction of a C–N bond through the Ritter reaction with simultaneous construction of a C–C bond through the Houben–Hoesch reaction as there would be a nitrilium ion intermediate (Scheme 1).
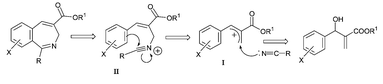 | ||
| Scheme 1 Schematic representation of synthetic strategy for 2-benzazepine derivatives. | ||
Accordingly, we first selected methyl 3-hydroxy-2-methylene-3-phenylpropanoate (1a), the Baylis–Hillman adduct obtained from methyl acrylate and benzaldehyde, as a substrate for performing the Ritter and Houben–Hoesch reactions with acetonitrile in the presence of methanesulfonic acid under various conditions. However this reaction did not proceed to the formation of the desired 2-benzazepine derivative but stopped at the allyl amide stage. The best results were obtained when methyl 3-hydroxy-2-methylene-3-phenylpropanoate (1a) (1 mmol) in acetonitrile (5 mL) was treated with methanesulfonic acid (3 mL) at 110 °C for 5 h, thus providing methyl (2E)-2-acetylaminomethyl-3-phenylprop-2-enoate (2a) in 72% isolated yield. We then prepared representative (E)-allyl amides (2b–f) via treatment of various Baylis–Hillman adducts (1b–d) with aceto-, propio-, and acrylonitriles (Scheme 2). With a view to understanding the stereochemical directive effects of the cyano group we also examined the reaction of 3-hydroxy-2-methylene-3-phenylpropanenitrile (3a) with acetonitrile under similar conditions, which provided (2Z)-2-acetylaminomethyl-3-phenylprop-2-enenitrile (4a) in 82% isolated yield. We then successfully transformed a representative class of Baylis–Hillman adducts (3b, 3c) into the corresponding (Z)-allyl amides (4b, 4c) (Scheme 2). This stereochemical reversal from ester group to cyano group is consistent with earlier reports on various transformations of the Baylis–Hillman adducts.9
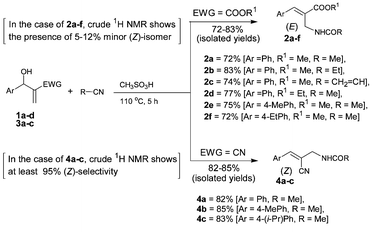 | ||
| Scheme 2 Stereoselective transformation of Baylis–Hillman adducts into (E)-allyl amides (2a–f) and (Z)-allyl amides (4a–c) under Ritter conditions.10,11 | ||
At this stage it occurred to us that the presence of electron donating group(s) on the aromatic ring might help in the construction of the C–C bond (via the Houben–Hoesch reaction) after construction of the C–N bond (via Ritter reaction) thus leading to the formation of 2-benzazepine derivatives (Scheme 3). Accordingly, we treated ethyl 3-hydroxy-2-methylene-3-(3-methoxyphenyl)propanoate (1e), the Baylis–Hillman adduct obtained from ethyl acrylate and 3-methoxybenzaldehyde, with acetonitrile in the presence of methanesulfonic acid under various conditions. We were pleased to isolate the expected 3-aza-2-methyl-5-ethoxycarbonyl-9-methoxybicyclo[5.4.0]undeca-1(7),2,5,8,10-pentaene (5) in 55% yield when 1e (2 mmol) in acetonitrile (5 mL) was treated with methanesulfonic acid (3 mL) at 150 °C for 6 h.12 We then extended this strategy to representative Baylis–Hillman adducts (1e–m) to provide the desired 2-benzazepine derivatives (6–15) in moderate to good yields via reaction with aceto- and propionitriles (Scheme 3, Table 1). The structures of 13 and 15 were also established by single crystal X-ray crystallography (Fig. 1).13
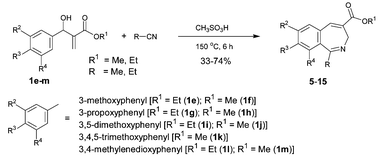 | ||
| Scheme 3 Facile one-pot synthesis of 2-benzazepine derivatives (5–15) involving novel tandem construction of C–N and C–C bonds.11 | ||
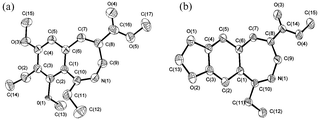 | ||
| Fig. 1 ORTEP diagrams of (a) 13 and (b) 15. | ||
| Alcohol (1e–m) |
| R1 (alkyl) | R (alkyl) | Product (5–15) | Yield (%) |
|---|---|---|---|---|---|
| a All the reactions were carried out on 2 mmol scale of Baylis–Hillman alcohols (1e–m) with methanesulfonic acid in alkanenitriles (5 mL) at 150 °C for 6 h. Yields are of the pure compounds obtained after column chromatography. All the compounds were fully characterized. | |||||
| 1e | 3-(MeO)Ph | Et | Me | 5 | 55 |
| 1e | 3-(MeO)Ph | Et | Et | 6 | 67 |
| 1f | 3-(MeO)Ph | Me | Et | 7 | 44 |
| 1g | 3-(PrO)Ph | Et | Et | 8 | 58 |
| 1h | 3-(PrO)Ph | Me | Et | 9 | 65 |
| 1i | 3,5-(MeO)2Ph | Et | Me | 10 | 70 |
| 1i | 3,5-(MeO)2Ph | Et | Et | 11 | 74 |
| 1j | 3,5-(MeO)2Ph | Me | Me | 12 | 72 |
| 1k | 3,4,5-(OMe)3Ph | Me | Et | 13 | 33 |
| 1l | 3,4-(OCH2O)Ph | Et | Et | 14 | 48 |
| 1m | 3,4-(OCH2O)Ph | Me | Et | 15 | 46 |
In conclusion, we have developed a novel strategy involving tandem construction of C–N and C–C bonds leading to a convenient one-pot procedure for the synthesis of 2-benzazepine derivatives from the Baylis–Hillman adducts. We have also described the stereoselective transformation of the Baylis–Hillman adducts into (E) or (Z)-allyl amides, thus demonstrating the efficacy of the Baylis–Hillman adducts as an important source for exploration of new reactions and stereoselective transformation methodologies.
We thank DST (New Delhi) for funding this project. We thank UGC (New Delhi) for recognizing our University of Hyderabad as a “University with Potential for Excellence (UPE)” and generous funding and also for a Special Assistance Program in Organic Chemistry in the School of Chemistry. TS thanks UGC (New Delhi) for his research fellowship. We also thank the National Single Crystal X-ray Facility in our School of Chemistry funded by DST (New Delhi). We thank Professor T. P. Radhakrishnan for helpful discussions regarding X-ray crystal structures.
Notes and references
- (a) J. A. May, R. K. Zeidan and B. M. Stoltz, Tetrahedron Lett., 2003, 44, 1203 CrossRef CAS; (b) C. Guillou, J-L. Beunard, E. Gras and C. Thal, Angew. Chem., Int. Ed., 2001, 40, 4745 CrossRef CAS; (c) C-K. Sha, A-W. Hong and C-M. Huang, Org. Lett., 2001, 3, 2177 CrossRef CAS; (d) J. Jin and S. M. Weinreb, J. Am. Chem. Soc., 1997, 119, 2050 CrossRef CAS; (e) J. M. Boente, L. Castedo, R. Cuadros, J. M. Saa, R. Suau, A. Perales, M. Martinez-Ripoll and J. Fayos, Tetrahedron Lett., 1983, 24, 2029 CrossRef CAS.
- (a) R. E. Johnson and C. A. Busacca (Sterling drug, Inc.), US Pat. 5,098,901; Chem. Abstr., 1992, 117, 7949j; (b) P. Croisier and L. Rodriguez (UCB S. A.), Ger. Offen. 2,733,868 and 2,733,869; Chem. Abstr., 1978, 88, 152455g and 152456h; (c) J. A. Meschino (McNeil Laboratories, Inc.), US Pat. 3,894,072; Chem. Abstr., 1975, 83, 114031e; (d) J. A. Meschino (McNeil Laboratories, Inc.), US Pat. 3,828,096; Chem. Abstr., 1974, 81, 136001f; (e) J. A. Meschino (McNeil Laboratories, Inc.), US Pat. 3,483,186; Chem. Abstr., 1970, 72, 121383x; (f) H. Fujimura and M. Hori (Takeda Chemical Industries, Ltd.), US Pat. 3,409,607; Chem. Abstr., 1969, 70, 77827c.
- (a) A. Kamimura, Y. Taguchi, Y. Omata and M. Hagihara, J. Org. Chem., 2003, 68, 4996 CrossRef CAS; (b) M. Nyerges, A. Viranyi, A. Pinter and L. Toke, Tetrahedron Lett., 2003, 44, 793 CrossRef CAS; (c) J. C. Martins, K. V. Rompaey, G. Wittman, C. Tomboly, G. Toth, N. De Kimpe and D. Tourwe, J. Org. Chem., 2001, 66, 2884 CrossRef CAS; (d) R. Gamez-Montano, M. I. Chavez, G. Roussi and R. Cruz-Almanza, Tetrahedron Lett., 2001, 42, 9 CrossRef CAS; (e) L. Ollero, L. Castedo and D. Dominguez, Tetrahedron Lett., 1998, 39, 1413 CrossRef CAS; (f) A. G. Griesbeck and H. Mauder, Angew. Chem., Int. Ed. Engl., 1992, 31, 73 CrossRef; (g) R. A. Holton, M. P. Sibi and W. S. Murphy, J. Am. Chem. Soc., 1988, 110, 314 CrossRef CAS.
- (a) D. Basavaiah and A. J. Rao, Chem. Commun., 2003, 604 RSC; (b) D. Basavaiah and T. Satyanarayana, Tetrahedron Lett., 2002, 43, 4301 CrossRef CAS; (c) D. Basavaiah and T. Satyanarayana, Org. Lett., 2001, 3, 3619 CrossRef CAS; (d) D. Basavaiah, M. Bakthadoss and S. Pandiaraju, Chem. Commun., 1998, 1639 RSC.
- (a) R. Bishop, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1991, vol. 6, p. 261 Search PubMed; (b) L. I. Krimen and D. J. Cota, Org. React., 1969, 17, 213 CAS; (c) K. V. Emelen, T. D. Wit, G. J. Hoornaert and F. Compernolle, Org. Lett., 2000, 2, 3083 CrossRef.
- (a) K. Hoesch, Ber. Dtsch. Chem. Ges., 1915, 48, 1122 CAS; (b) J. Houben, Ber. Dtsch. Chem. Ges., 1926, 59, 2878; (c) P. E. Spoerri and A. S. DuBois, Org. React., 1949, 5, 387 CAS; (d) W. Ruske, in Freidel–Crafts and Related Reactions, ed. G. A. Olah, Wiley-Interscience, New York, 1964, vol. III, part I, ch. 32 Search PubMed; (e) M. Yato, T. Ohwada and K. Shudo, J. Am. Chem. Soc., 1991, 113, 691 CrossRef CAS; (f) Y. Sato, M. Yato, T. Ohwada, S. Saito and K. Shudo, J. Am. Chem. Soc., 1995, 117, 3037 CrossRef CAS; (g) R. Kawe-eki, A. P. Mazurek, L. Kozerski and J. K. Maurin, Synthesis, 1999, 751 CrossRef.
- (a) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS; (b) E. Ciganek, in Organic Reactions, ed. L. A. Paquette, John Wiley & Sons, New York, 1997, vol 51, p. 201 Search PubMed; (c) D. Basavaiah, P. Dharma Rao and R. Suguna Hyma, Tetrahedron, 1996, 52, 8001 CrossRef CAS; (d) S. E. Drewes and G. H. P. Roos, Tetrahedron, 1988, 44, 4653 CrossRef CAS.
- (a) V. K. Aggarwal, I. Emme and S. Y. Fulford, J. Org. Chem., 2003, 68, 692 CrossRef CAS; (b) K.-S. Yang, W.-D. Lee, J.-F. Pan and K. Chen, J. Org. Chem., 2003, 68, 915 CrossRef CAS; (c) W. P. Almeida and F. Coelho, Tetrahedron Lett., 2003, 44, 937 CrossRef CAS; (d) H. Kraiem, M. I. Abdullah and H. Amri, Tetrahedron Lett., 2003, 44, 553 CrossRef CAS; (e) M. Shi and Y-M. Xu, Angew. Chem., Int. Ed., 2002, 41, 4507 CrossRef CAS; (f) C. Yu and L. Hu, J. Org. Chem., 2002, 67, 219 CrossRef CAS; (g) L.-C. Wang, A. L. Luis, K. Agapiou, H.-Y. Jang and M. J. Krische, J. Am. Chem. Soc., 2002, 124, 2402 CrossRef CAS; (h) S. A. Frank, D. J. Mergott and W. R. Roush, J. Am. Chem. Soc., 2002, 124, 2404 CrossRef CAS; (i) R. Räcker, K. Döring and O. Reiser, J. Org. Chem., 2000, 65, 6932 CrossRef; (j) G. Li, J. Gao, X. Wei II and M. Enright, Org. Lett., 2000, 2, 617 CrossRef CAS; (k) A. G. M. Barrett, A. S. Cook and A. Kamimura, Chem. Commun., 1998, 2533 RSC.
- (a) D. Basavaiah and P. K. S. Sarma, Chem. Commun., 1992, 955 RSC; (b) D. Basavaiah, M. Krishnamacharyulu, R. Suguna Hyma and S. Pandiaraju, Tetrahedron Lett., 1997, 38, 2141 CrossRef CAS; (c) H. J. Lee, M. R. Seong and J. N. Kim, Tetrahedron Lett., 1998, 39, 6223 CrossRef.
- See ESI† for E/Z of the amides.
- A plausible mechanism for the formation of allyl amides and 2-benzazepines is presented in the ESI†.
- A temperature of 150 °C is necessary as at 110 °C 2-benzazepine (5) is not formed. Also in the case of 1a, even at 150 °C there is no formation of the corresponding 2-benzazepine derivative.
- Crystal data for 13: C17H21NO5; M, 319.35; crystal colour, habit: light yellow, rectangular; crystal dimensions, 0.5 × 0.48 × 0.24 mm; crystal system, monoclinic; lattice type, primitive; a = 10.669(6), b = 13.041(8), c = 12.062(9) Å; β = 93.18(5)°; V = 1675.6(18) Å3; space group, P21/a : b3 (No. 14); Z = 4; μ = 0.093 mm−1; Dcalcd = 1.266 g cm−3; F000 = 680; λ(Mo Kα) = 0.71073 Å; R = 0.0569, wR2 = 0.1845. Crystal data for 15: C15H15NO4; M, 273.28; crystal colour, habit: light yellow, rectangular; crystal dimensions, 0.47 × 0.42 × 0.40 mm; crystal system, monoclinic; lattice type, primitive; a = 8.096(5), b = 16.436(8), c = 10.159(8) Å; β = 98.73(5)°; V = 1336.1(15) Å3; space group, P21/a : b3 (No. 14); Z = 4; μ = 0.099 mm−1; Dcalcd = 1.359 g cm−3; F000 = 576; λ(Mo Kα) = 0.71073 Å; R = 0.0441, wR2 = 0.1081. CCDC 211673 and 211674. See http://www.rsc.org/suppdata/cc/b3/b310550d/ for crystallographic data in CIF or other electronic format.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, analytical, spectral data for all 2-benzazepine derivatives (5–15) and allyl amides (2a–f, 4a–c). Stereochemical assignment for 2a–f and 4a–c. See http://www.rsc.org/suppdata/cc/b3/b310550d/ |
| This journal is © The Royal Society of Chemistry 2004 |