Controlled growth of calcium carbonate by poly(ethylenimine) at the air/water interface
Hyoung
Kun Park
,
Inhyung
Lee
and
Kwan
Kim
*
Laboratory of Intelligent Interfaces, School of Chemistry and Molecular Engineering and Center for Molecular Catalysis, Seoul National University, Seoul 151-472, Korea. E-mail: kwankim@plaza.snu.ac.kr
First published on 21st November 2003
Abstract
Two metastable calcium carbonate polymorphs, hemispherical vaterite and needle-like aragonite, are selectively formed at the air/water interface by the mediation of poly(ethyleneimine) (with molecular weights of 25000 and 2000, respectively) dissolved in supersaturated calcium bicarbonate solution.
Thermodynamically, the least stable phase of calcium carbonate is vaterite followed by aragonite and calcite.1 These three polymorphs are in fact encountered in nature, and their structural selectivity has long been an intriguing aspect of biomineralization.2 Recent studies suggest that specific biological macromolecules are involved in controlling calcium carbonate polymorphism.3 In laboratories, vaterite (or aragonite) can be prepared only in the presence of specific additives. For instance, anionic dendrimers induce crystallization of spherical vaterite,4 and double hydrophilic block copolymers lead to the precipitation of spherical or hollow shell vaterite (or calcite) particles.5 Thin films of aragonite can be deposited on chitosan matrices by the cooperation of chitosan, poly(aspartate) and MgCl2 in supersaturated calcium bicarbonate solution.6 Here, we report on the selective growth of vaterite or aragonite at the air/water interface by the use of poly(ethylenimine) (PEI) dissolved in a supersaturated calcium bicarbonate solution.
PEI has a highly branched structure with a distribution of primary, secondary, and tertiary amine groups in the ratio of 1∶2∶1.7 One of the unique characteristics of PEI is its ready formation of complexes with anionic surfactants or DNA due to its high charge density.8 Similarly, PEI can take advantage of its multiple cationic sites to bind anions (carbonate ions) for the subsequent growth of calcium carbonate crystals.
In fact, even in the absence of PEI, calcium carbonate can crystallize from supersaturated calcium bicarbonate solution by the slow evaporation of CO2.† The crystals floating at the air/water interface are quite irregular, with noticeable size distribution, and can be identified as consisting mostly of calcite and vaterite. In contrast, the addition of PEI into supersaturated calcium bicarbonate solution results in controlled crystallization. Not only do the crystals grow exclusively at the air/water interface, but the polymorphs of the crystals are affected by the molecular weight of PEI; in this experiment, PEI with molecular weights of 25000, 2000, or 700 (denoted as PEI25000, PEI2000, and PEI700, respectively) has been added to a final concentration of (2.2 × 10−2)–(4.5 × 10−1) g L−1. In the presence of PEI25000, circularly shaped crystals with diameters of 5–10 µm grow at the air/water interface. According to SEM images, the crystals viewed from the air-side are quite flat, while the surfaces on the water-side are hemispherical and rough; see Figs. 1a and 1b, respectively. More enlarged SEM images reveal that the water-side surfaces are comprised of small particles fused together with diameters smaller than 100 nm (see the inset of Fig. 1d). In the presence of PEI2000, strongly aggregated crystals are formed at the air/water interface; (see Figs. 1c and 1d that show the SEM images viewed from the air- and water-side, respectively). Shown inset in Fig. 1d, the enlarged SEM image clearly indicates the formation of needle-like crystals at the air/water interface. The needle-like crystals form as large as ∼1 × 2 µm. In the presence of PEI700, however, polyhedral or leaflet-like crystals are formed with much larger sizes (30∼50 µm) and irregular shapes and distributions (data not shown).
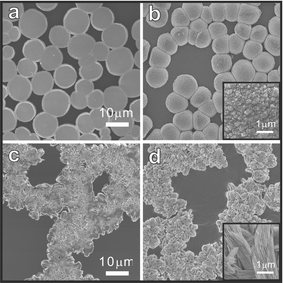 | ||
| Fig. 1 Scanning electron micrographs of two metastable CaCO3 polymorphs formed at the air/water interface from supersaturated calcium bicarbonate solutions in the presence of PEI25000 (‘a’ and ‘b’) and PEI2000 (‘c’ and ‘d’): ‘a’ and ‘c’ are air-side views and ‘b’ and ‘d’ are water-side views. Insets are magnified, water-side images. | ||
Figs. 2a, 2b, and 2c show the XRD patterns for the crystals formed in the presence of PEI25000, PEI2000 and PEI700, respectively. It is evident that the XRD peaks in Fig. 2a are exclusively due to vaterite while the peaks in Fig. 2b are due to aragonite.9 In contrast, the XRD peaks in Fig. 2c are due to a mixture of calcite and vaterite; according to Raman spectroscopy, polyhedral crystals in the mixture are identified to be calcite while leaflet-like crystals are vaterite (data not shown). A control experiment conducted in the absence of PEI resulted in similarly mixed crystals with a comparable composition (data not shown). These XRD data indicate that metastable CaCO3 crystals (vaterite/aragonite) can be stabilized by PEI of a higher molecular weight. It is also remarkable that aragonite can be formed even in the complete absence of any inorganic metal ions, such as Mg2+. Polymer-mediated aragonite formation is of great importance, not only from the scientific point of view, but also from the technological point of view of fabricating very hard organic/inorganic hybrid materials.2
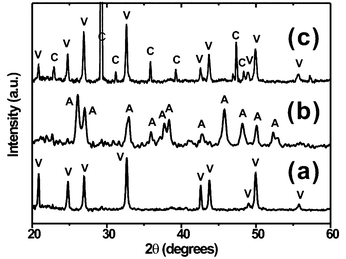 | ||
| Fig. 2 X-Ray diffraction patterns of CaCO3 crystals formed at the air/water interface in the presence of (a) PEI25000, (b) PEI2000, and (c) PEI700; vaterite (V), aragonite (A), and calcite (C). | ||
The detailed mechanism of how PEI controls the growth of specific polymorphs of CaCO3 is a matter of conjecture. However, carbonate ions in equilibrium with bicarbonate species must bind to protonated amine groups of PEI (pKa = 10∼11) in the initial stage of the crystallization of calcium carbonate. To obtain information indirectly on the carbonate–amine interaction at the air/water interface where crystallization takes place, we have measured the surface pressure–area (π–A) isotherms of stearic acid (STA) on a supersaturated calcium bicarbonate subphase (see Fig. 3-2); for comparative purposes, the isotherms measured on a pure water subphase are shown in Fig. 3-1. On a pure water subphase, the isotherm clearly exhibits the gas/solid and solid/solid-phase transition upon increasing the surface pressure. As PEI is added into water, the isotherm of STA exhibits somewhat expanded features but it still shows the gas/solid phase transition (see Figs. 3-1b, 1c, and 1d). However, on a supersaturated calcium bicarbonate subphase, highly expanded isotherms are obtained specifically in the presence of PEI2000 and PEI25000 (see Figs. 3-2c and 3-2d);7 under the latter conditions, the onsets of the first pressure increases occur at ∼0.7 and ∼0.8 nm2 per molecule and wide plateau regions are observed at ∼3 mN m−1 and at ∼10 mN m−1, respectively. The plateau regions must correspond to domains where liquid-condensed (LC) and liquid-expanded (LE) phases can coexist. This points to the propensity of PEI molecules near the air/water interface to interact efficiently with the carboxylate groups of STA. It is as if the headgroup of STA becomes bulkier and heavier with any increase in the molecular weight of PEI.10 It should be mentioned that the isotherm of STA obtained on a CaCl2 (9 mM) subphase (data not shown) differs little from that on a pure water subphase (Fig. 3-1), however; even in the presence of PEI25000, the isotherm of STA exhibits the gas/solid phase transition. These observations clearly indicate that PEI and carbonate (as well as bicarbonate) interact efficiently at the air/water interface. As indicated by the isotherm of STA, their interaction becomes greater in proportion to the increasing molecular weight of PEI.
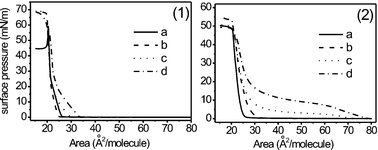 | ||
| Fig. 3 Pressure–area isotherms of stearic acid on pure water subphase (1) and on supersaturated calcium bicarbonate subphase (2) in the presence of (a) no additive, (b) PEI700, (c) PEI2000, and (d) PEI25000 at 2.2 × 10−2g L−1. | ||
In principle, inorganic nuclei can be formed on organic surfaces by lowering the activation energy of nucleation (ΔG‡) through interfacial recognition.11 Different interactions between organic surfaces and inorganic ions may then create an ensemble of nucleation profiles of ΔG‡ such as to make kinetic control of crystal polymorphs possible.12 In light of this, gradually increasing interactions (Fig. 3-2a→d) between the amine groups of PEI and a carbonate would presumably induce the formation of less stable polymorphs. In the presence of PEI that demonstrates the LC-LE coexistence features for STA (Fig. 3-2c,d), only a single polymorph (vaterite or aragonite) is nucleated at the air/water interface, while mixed polymorphs, including calcite, grow under the conditions in which the gas-to-solid transition is observed (Fig. 3-2a,b). Once thermodynamically unstable vaterite or aragonite are formed over the highly branched polymeric template, they are stable for extended periods of time (∼3 days), persisting in competition with the thermodynamic product, calcite. In the absence of any additive, vaterite readily transforms into stable calcite via a solvent-mediated process.13
Another notable feature of this process is the persistent formation of crystals at the air/water interface. In general, it is well known that an inhibition of crystallization from solution is guaranteed by the flexibility of polyelectrolyte in solution.14 The exclusive growth of crystals at the air/water interface is also associated with continual CO2 outgassing, resulting in local supersaturation levels remaining low. Small crystals formed in the bulk may gradually dissolve towards maturation of larger ones at the air/water interface according to the Ostwald ripening process.2
This work can be compared with the literature that describes how acidic macromolecules have been exclusively used in calcium carbonate crystallization on the grounds that biological organic–inorganic structures contain proteins with glutamic or aspartic acid residues,3 and how polyacrylic acid is industrially used to prevent calcium carbonate from scaling.2 This work suggests that basic polymers can also be useful templates for the growth of functional crystals. It is noteworthy that thermodynamically unstable minerals can be grown and stabilized using simple polymers like PEI. We are examining whether size, shape, and polymorphism can be controlled for other crystals using PEI.
This work was supported by the Ministry of Health & Welfare (Korea Health 21 R&D Project, 01-PJ11-PG9-01NT00-0023) of the Republic of Korea.
Notes and references
- H. A. Lowenstam and S. Weiner, On Biomineralization, Oxford University Press, New York, 1989 Search PubMed.
- Biomimetic Materials Chemistry, ed. S. Mann, VCH, New York 1996 Search PubMed.
- (a) G. Falini, S. Albeck, S. Weiner and L. Addadi, Science, 1996, 271, 67; (b) A. M. Belcher, X. H. Wu, R. J. Christensen, P. K. Hansma, G. D. Stucky and D. E. Morse, Nature, 1996, 381, 56 CrossRef CAS.
- K. Naka, Y. Tanaka and Y. Chujo, Langmuir, 2002, 18, 3655 CrossRef CAS.
- H. Cölfen and M. Antonietti, Langmuir, 1998, 14, 582 CrossRef.
- A. Sugawara and T. Kato, Chem. Commun., 2000, 487 RSC.
- M. J. Hwang and K. Kim, Langmuir, 1999, 15, 3563 CrossRef CAS.
- A. F. Thunemann and J. Beyermann, Macromolecules, 2000, 33, 6878 CrossRef.
- I. Lee, S. W. Han, H. J. Choi and K. Kim, Adv. Mater., 2001, 13, 1617 CrossRef CAS.
- M. H. Lee, T. H. Ha and K. Kim, Langmuir, 2002, 18, 2117 CrossRef CAS.
- H. Cölfen and S. Mann, Angew. Chem. Int. Ed., 2003, 42, 2350 CrossRef.
- S. Mann, D. D. Archibald, J. M. Didymus, T. Douglas, B. R. Heywood, F. C. Meldrum and N. F. Reeves, Science, 1993, 261, 1286 CAS.
- A. Lopezmacipe, J. Gomezmorales and R. Rodriguezclemente, J. Cryst. Growth, 1996, 166, 1015 CrossRef CAS.
- L. Addadi and S. Weiner, Angew. Chem., Int. Ed. Engl., 1992, 31, 153 CrossRef.
- Y. Kitano, Bull .Chem. Soc. Jpn., 1962, 35, 1973 CAS.
Footnote |
| † Supersaturated calcium bicarbonate solution was prepared by bubbling CO2 gas into Milli-Q deionized water in the presence of CaCO3 (2g/4L) for 4 hours.15 Excess solid CaCO3 was removed by filtering, and the filtrate was purged with CO2 for another 30 min. The solution thus prepared had been adjusted to a pH of 6.2 ± 0.1 and calcium concentration of ∼9 mM as determined by ICP-AES. All the crystal growth was conducted at 25 °C for 20 hours. Then, crystals floating at the air/water interface were transferred to a glass or Si wafer and analyzed by SEM (JSM-6700F) and XRD (Rigaku DMAXIIA). The pressure–area isotherm of stearic acid was measured, after spreading its chloroform solution (2 mM, 80 µL) at the air/water interface, using a microprocessor-controlled film balance (KSV 3000 Langmuir balance). |
| This journal is © The Royal Society of Chemistry 2004 |
