Hybrid SAM/LB device structures: manipulation of the molecular orientation for nanoscale electronic applications
Geoffrey J. Ashwell* and Martial Berry
The Nanomaterials Group, Cranfield University, Cranfield, UK MK43 0AL. E-mail: g.j.ashwell@cranfield.ac.uk; Fax: 01234-754684; Tel: 01234-752452
First published on 5th November 2004
Abstract
Asymmetric current–voltage characteristics have resulted from hybrid SAM/LB structures of squaraine dyes in which the donor-acceptor-donor (D-A-D) molecules are aligned vertically in the self-assembled monolayer and horizontally in the Langmuir–Blodgett film: the separate layers exhibit symmetrical I–V characteristics and the altered behaviour of the hybrid bilayer is attributed to orientation-induced molecular rectification.
Molecular electronics represents the ultimate challenge in device miniaturisation and the molecular diode,1 an organic counterpart of the pn junction, is once more the subject of intense theoretical2–4 and experimental5–12 interest. There are few reported examples, with only seven different bridged donor–acceptor chromophores so far exhibiting rectification when contacted by non-oxidisable electrodes: two neutral6,7 and three cationic8–10 D-π-A dyes with substituent groups for self-assembly and/or LB deposition and two chevron-shaped D-π-A-π-D dyes11,12 that align in LB films without requiring long substituent alkyl tails. At forward bias, electrons tunnel from the electrode to the lowest unoccupied molecular orbital of the acceptor at one end of the device and from the highest occupied molecular orbital of the donor to the electrode on the opposite side. At reverse bias, there is an energy mismatch of the Fermi and molecular levels, which induces the electrical asymmetry. In this communication, we now report unexpected behaviour and demonstrate that rectification may be induced by squaraine molecules when their orientations are orthogonal in the separate layers of a hybrid SAM/LB structure. The discovery has important implications for molecular scale electronics and the design of component molecules.
The precusor for molecular self-assembly was synthesised by reacting 1-(3-acetylsulfanylpropyl)lepidinium iodide and 2,3,3-trimethylindolenine with squaric acid (1 : 1 : 1 ratio) in propan-2-ol containing a few drops of triethyl orthoformate, the mixture being heated at reflux for 72 h. The solvent was removed in vacuo and the product extracted into chloroform. The required squaraine was isolated by flash chromatography on silica gel, eluting with chloroform and a variable ratio of chloroform to methanol. Preparative plate chromatography yielded metallic green crystals of 2-[1-(3-acetylsulfanylpropyl)quinolin-4-ylidenemethyl]-4-[3,3-dimethylindolenin-2-ylidenemethyl]squaraine (1), which provided satisfactory analytical data.†
SAMs were fabricated by immersing gold-coated substrates in an ethanol solution of the precursor (0.1 mg cm−3) to which a few drops of ammonium hydroxide had been added to displace the acetyl group. Immersion of the substrates for several short periods totalling ca. 2 h with thorough rinsing to remove physisorbed dye provided optimum conditions for chemisorption. A Sauerbrey13 analysis of the frequency change, following deposition on gold-coated 10 MHz quartz crystals, provided the molecular area, and a two-layer analysis of surface plasmon resonance (SPR) data, obtained using a Kretschmann configuration,14 provided the thickness. Dimensions of 0.45 ± 0.05 nm2 molecule−1 and 1.5 ± 0.2 nm approximate to the van der Waals cross-section and length respectively and suggest that the chromophores are almost upright. Electrical characterisation was peformed on self-assembled films on gold-coated highly oriented pyrolytic graphite (HOPG) using a Nanoscope IV scanning tunnelling microscope with the films contacted by PtIr tips. The SAMs exhibit symmetrical I–V characteristics, the data being shown in Fig. 1, but there is molecular asymmetry, the two donor groups being different with one connected via an Au–S–C3H6 link to the substrate electrode. This is insufficient to induce rectification.
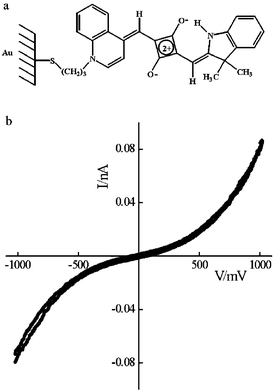 | ||
| Fig. 1 SAM: (a) molecular structure of 1 and (b) I–V characteristics of the monolayer contacted by a PtIr tip. | ||
The LB film-forming dye, 2,4-bis[N-methyl-N-(hexylamino)phenyl]squaraine (2) was synthesised as previously described.15 Films were obtained by spreading a dilute chloroform solution of the dye (0.1 mg cm−3) onto the pure water subphase of a Nima Technology LB trough and then, after ca. 10 min, compressing the floating monolayer at 50 mm2 s−1 and depositing the film on the upstroke at 0.1 mm s−1 and a surface pressure of 10 mN m−1. Films transferred to 10 MHz quartz crystals exhibited a frequency change that is consistent with a mean area of 0.90 nm2 molecule−1 and which approximates to the van der Waals area of the long edge of the squaraine. A thickness of 0.819 nm was obtained from grazing incidence X-ray synchrotron diffraction studies and also verified by an SPR analysis, the latter providing a thickness of 0.7 ± 0.2 nm. These dimensions confirm that the molecules align with their long axes parallel to the substrate, which is a common feature of the butyl to dodecyl derivatives of the 2,4-bis[N-methyl-N-(alkylamino)phenyl]squaraines15,16 but is quite unusual for LB film-forming dyes. The chromophores are centric17 and, as above, LB films of these dyes on gold-coated HOPG also exhibit symmetrical I–V characteristics.
The electrical properties may be manipulated by depositing an LB monolayer onto the SAM to induce different molecular orientations in the upper and lower layers of the hybrid device. The thickness of the SAM/LB structure, determined from SPR studies, is consistent with the sum of the separate monolayer thicknesses (SAM, 1.5 nm; LB, 0.8 nm) and confirms that the different molecular orientations are maintained in the bilayer. However, the I–V characteristics are altered and the hybrid structure exhibits a rectification ratio of ca. 12 at ±1 V (Fig. 2). The electrical asymmetry is tentatively explained by different orientations of the molecules with respect to the two electrodes: the D-A-D chromophore is horizontally aligned in the upper layer, thereby placing its long axis in contact with the PtIr tip, and upright in the lower layer where it projects a terminal donor towards the substrate electrode. At forward bias, which corresponds to the positive quadrant of the I–V plot, electrons tunnel via the molecules from the contacting tip to the substrate electrode. It suggests that the horizontally aligned chromophore is a more likely electron acceptor than the heterocyclic donor on the opposite side of the device, the assignment being loosely consistent with the Aviram and Ratner model,1 albeit modified for the differently aligned components in the hybrid bilayer.
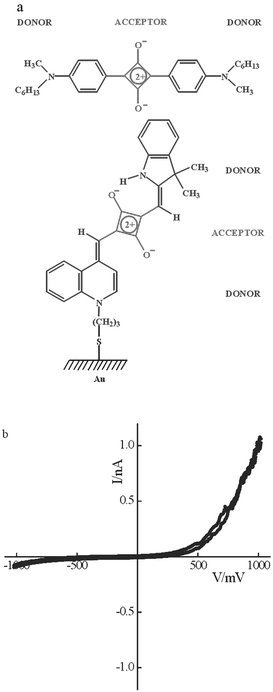 | ||
| Fig. 2 Hybrid SAM/LB device: (a) molecular structures indicating alignment within the SAM of dye 1 and LB overlay of dye 2; (b) typical I–V characteristics of the hybrid bilayer contacted by a PtIr tip. The polarity corresponds to the substrate electrode and at forward bias, which corresponds to the positive quadrant of the I–V plot, electrons tunnel in the direction of tip to substrate. | ||
Other self-assembling analogues form part of an on-going investigation and exhibit rectification when squaraine molecules are aligned differently at the interfaces with the two electrodes. In each case, the direction of electron tunnelling at forward bias is from the electrode adjacent to the horizontally aligned chromophores. Furthermore, consistent I–V curves and polarities for rectification have been obtained for films contacted by alkylthiolate-coated mercury drop electrodes (CnH2n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–S–Hg) when interrogated by using a Keithley source and electrometer. The behaviour is reproducible and the different measurement techniques provide evidence that the electrical asymmetry is an inherent property of the hybrid SAM/LB structure.
1–S–Hg) when interrogated by using a Keithley source and electrometer. The behaviour is reproducible and the different measurement techniques provide evidence that the electrical asymmetry is an inherent property of the hybrid SAM/LB structure.
In conclusion, I–V characteristics of SAMs and LB films of squaraine dyes are symmetrical when the chromophores are aligned horizontally or vertically but exhibit significant electrical asymmetry when oriented differently in the separate layers of hybrid SAM/LB structures. We suggest that rectification arises from the packing arrangement, whereby a vertically aligned molecule projects its donor towards one electrode and a horizontally aligned molecule has the long axis of its D-A-D chromophore in contact with the other.
Acknowledgements
We are grateful to Professor Colin Kennard for the GIXD data, Robert Stokes for technical assistance, the EPSRC for financial support and the EPSRC National Mass Spectrometry Service for providing MS data on the squaraine dyes.References
- A. Aviram and M. A. Ratner, Chem. Phys. Lett., 1974, 29, 277 CrossRef CAS.
- K. Stokbro, J. Taylor and M. Brandbyge, J. Am. Chem. Soc., 2003, 125, 3674 CrossRef CAS.
- S. Ami, M. Hliwa and C. Joachim, Chem. Phys. Lett., 2003, 367, 662 CrossRef CAS.
- A. Troisi and M. A. Ratner, J. Am. Chem. Soc., 2002, 124, 14528 CrossRef CAS.
- T. Reda, A. F. Collings, C. Barton and P. Lukins, J. Phys. Chem. B, 2003, 107, 13774 CrossRef CAS.
- G. J. Ashwell, A. Chwialkowska and L. R. H. High, J. Mater. Chem., 2004, 14, 2389 RSC; G. J. Ashwell, R. Hamilton and L. R. H. High, J. Mater. Chem., 2003, 13, 1501 RSC; T. Xu, I. R. Peterson, M. V. Lakshmikantham and R. M. Metzger, Angew. Chem., Int. Ed., 2001, 40, 1749 CrossRef CAS; R. M. Metzger, T. Xu and I. R. Peterson, J. Phys. Chem. B, 2001, 105, 7280 CrossRef CAS; J. R. Sambles, N. Okazaki, M. J. Jory and G. J. Ashwell, Appl. Phys. Lett., 2002, 81, 2300 CrossRef.
- G. J. Ashwell, A. Chwialkowska and L. R. H. High, J. Mater. Chem., 2004, 14, 2848 RSC.
- G. J. Ashwell, W. D. Tyrrell and A. J. Whittam, J. Am. Chem. Soc., 2004, 126, 7102 CrossRef CAS; G. J. Ashwell, W. D. Tyrrell and A. J. Whittam, J. Mater. Chem., 2003, 13, 2855 RSC; G. J. Ashwell, D. S. Gandolfo and R. Hamilton, J. Mater. Chem., 2002, 12, 416 RSC.
- G. J. Ashwell and D. S. Gandolfo, J. Mater. Chem., 2001, 11, 246 RSC; G. J. Ashwell and D. S. Gandolfo, J. Mater. Chem., 2002, 12, 411 RSC.
- G. J. Ashwell and A. Mohib, unpublished data. SAMs of 1-(10-mercaptodecyl)-4-[2-(4-methoxy-naphthalen-1-yl)-vinyl]-quinolinium iodide exhibit rectification ratios of ca. 30 at ±1 V.
- G. J. Ashwell and M. A. Amiri, J. Mater. Chem., 2002, 12, 2181 RSC; J. W. Baldwin, R. R. Amaresh, I. R. Peterson, W. J. Shumate, M. P. Cava, M. A. Amiri, R. Hamilton, G. J. Ashwell and R. M. Metzger, J. Phys. Chem. B, 2002, 106, 12158 CrossRef CAS.
- G. J. Ashwell and M. Sujka, unpublished data. LB monolayers of N-butyl-2,6-bis[2-{4-(2-(4-dibutylaminophenyl)vinyl)phenyl}vinyl]pyridinium iodide exhibit rectification ratios of ca. 16 at ± 1 V.
- G. Sauerbrey, Z. Phys., 1959, 155, 206 CAS.
- E. Kretschmann, Z. Phys., 1971, 241, 313 CAS.
- G. J. Ashwell, M. P. S. Roberts, N. D. Rees, G. S. Bahra and C. R. Brown, Langmuir, 1998, 14, 5279 CrossRef CAS.
- G. J. Ashwell, G. Jefferies, D. G. Hamilton, D. E. Lynch, M. P. S. Roberts, N. D. Rees, G. S. Bahra and C. R. Brown, Nature, 1995, 375, 385 CrossRef CAS.
- G. J. Ashwell, G. S. Bahra, C. R. Brown, D. G. Hamilton, C. H. L. Kennard and D. E. Lynch, J. Mater. Chem., 1996, 6, 23 RSC.
Footnote |
† Analytical data for 1: yield, 20%; mp 220–222 °C. λmax
(CHCl3): 755 nm. Found: C, 72.1; H, 5.5; N, 5.5%. C30H28N2O3S requires C, 72.56; N, 5.68; H, 5.64%. 1H NMR (CDCl3): δH 1.44 (s, 6H, CH3); 2.15 (quintet, J 7.1, 2H, SCH2CH2CH2N); 2.38 (s, 3H, CH3CO); 2.94 (t, J 7.0, 2H, SCH2); 4.21 (t, J 7.3, 2H, CH2N); 5.49 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H); 6.48 (s, 1H, C C–H); 6.48 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H); 7.01 (t, J 7.5, 1H, Ar–H); 7.07 (d, J 8.0, 1H, Ar–H); 7.22 (d, J 7.7, 1H, Ar–H); 7.39–7.44 (m, 3H, Ar–H); 7.63 (t, J 8.1, 1H, Ar–H); 8.24 (d, J 9.9, 1H, Ar–H); 8.65 (d, J 6.4, 1H, Ar–H); 8.76 (d, J 8.2, 1H, Ar–H); 13.0 (s, 1H, NH) ppm. m/z
(FAB): 497 [M + H]+. C–H); 7.01 (t, J 7.5, 1H, Ar–H); 7.07 (d, J 8.0, 1H, Ar–H); 7.22 (d, J 7.7, 1H, Ar–H); 7.39–7.44 (m, 3H, Ar–H); 7.63 (t, J 8.1, 1H, Ar–H); 8.24 (d, J 9.9, 1H, Ar–H); 8.65 (d, J 6.4, 1H, Ar–H); 8.76 (d, J 8.2, 1H, Ar–H); 13.0 (s, 1H, NH) ppm. m/z
(FAB): 497 [M + H]+. |
| This journal is © The Royal Society of Chemistry 2005 |
