Polyelectrolyte-mediated synthesis and self-assembly of silicalite nanocrystals into linear chain superstructures
Kensuke
Aoki
ab and
Stephen
Mann
a
aSchool of Chemistry, University of Bristol, Bristol, UK BS8 1TS. E-mail: s.mann@bristol.ac.uk
bCentral Technology Laboratory, Asahi Kasei Corporation, 2-1 Samejima, Fuji, Shizuoka 416-8501, Japan
First published on 12th November 2004
Abstract
Hydrothermal synthesis in the presence of the cationic polyelectrolyte poly(diallydimethylammonium chloride) produces silicalite nanoparticles with uniform shape and size that spontaneously self-assemble into linear chains co-aligned along the crystallographic [010] direction.
Zeolites are useful functional materials based on precise arrangements of nanopores generated by their crystalline structures. They are widely used in large quantities as catalysts, molecular sieves, drying and absorbent agents, and ion exchange materials.1 Although only relatively few zeolite compositions are used in large-scale processes, specifically tailored and hierarchically structured zeolite-like materials could provide a new generation of advanced functional materials in fields such as electronics, photonics and information engineering.2 In this context, the use of nanoparticle building blocks for the construction of higher-order architectures—nanotectonics—is a promising chemically based approach to hierarchical, multi-functional materials.3 For example, nanoparticles of silicalite-1, which has an aluminium-free ZSM-5 structure, have been used as preformed building blocks to construct organized materials such as hollow spheres,4 macroporous fibres,5 sponges6 and foams.7 Similarly, micron-sized zeolite crystals have been used for the fabrication of organized 2-D arrays,8 complex hollow microspheres,9 and macroporous frameworks.10 In contrast, there have been few reports on the spontaneous one-step assembly of nanoparticle-based zeolite superstructures by direct synthesis. Recently, Kremer et al. showed that nanoslabs of silicalite-1 produced during the initial stages of surfactant-mediated synthesis spontaneously assembled into a multi-scale porous superstructure.11 Herein, we report the coupled synthesis and self-assembly of stable chains of crystallographically aligned silicalite-1 nanocrystals in the presence of the cationic polyelectrolyte, poly(diallydimethylammonium chloride) (PDAC).
Synthesis of silicalite-1 nanocrystals was carried out at 150 °C for 24 hours by hydrothermal treatment of mixtures of tetraethylorthosilicate (TEOS) and tetrapropylammonium hydroxide (TPAOH) in the presence or absence of PDAC (Mr = 1–2 × 105).12 In the absence of PDAC, randomly dispersed prismatic crystals of silicalite were observed by scanning electron microscopy (SEM) (Fig. 1a) The control crystals were in the form of flattened pseudo-hexagonal prisms of mean width and thickness of ca.180 nm and 110 nm, respectively. In contrast, addition of PDAC at a TEOS : PDAC molar ratio = 1 : 1.5 × 10−6 in the starting solution produced linear chain-like superstructures that consisted of up to 20 interconnected silicalite nanocrystals, which had the same structure and morphology but larger mean size compared with those formed in the absence of PDAC (Fig. 1b).13 FTIR spectra and TGA profiles did not confirm the presence of significant amounts of the PDAC polymer associated with the washed chains.14 The chains were resistant to repeated ultrasonication and centrifugation during the washing procedure, although extensive ultrasonication did result in partial chain fragmentation. Moreover, the chain-like superstructures and their constituent prismatic silicalite nanoparticles were retained after removal of the TPAOH template by heating in air at 550 °C (Fig. 1c).15
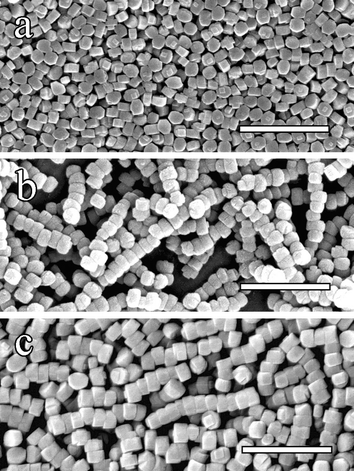 | ||
| Fig. 1 SEM images showing: (a) silicalite-1 nanocrystals prepared in the absence of PDAC, (b) PDAC-mediated silicalite chain superstructures, and (c) silicalite nanoparticle chains after calcination at 550 °C for 24 hours, followed by redispersion in ethanol (scale bars = 1 µm). | ||
TEM images and corresponding electron diffraction patterns indicated that the co-assembled silicalite nanocrystals were preferentially aligned with the crystallographic b direction parallel to the chain axis (Fig. 2). In addition, electron diffraction patterns indicated that the crystals were often arranged in the chains such that the a and c axes of adjacent crystals were mutually aligned normal to the chain axis (Fig. 2b). Stacking of the crystals therefore involved strong interactions between the relatively large and smooth {010} faces of neighbouring crystals. These results were consistent with XRD patterns recorded from samples of chains air-dried onto silicon substrates, which showed a reduced peak intensity for the silicalite (0 ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/char_0030_0332.gif) 0) reflection (data not shown). Time-dependent studies16 indicated that discrete silicalite nanocrystals with irregular disk-like morphology were present in samples taken up to 4 h after the start of the hydrothermal reaction (Fig. 3a). The assembly of intact chains occurred between 4 and 6 hours after the start of hydrothermal treatment, and was associated with transformation of the precursor particles into well-defined pseudo-hexagonal prismatic tablets (Fig. 3b). Once formed, the concentration of chains, as well as the number of nanocrystals per chain did not significantly increase with time (Fig. 3c).
0) reflection (data not shown). Time-dependent studies16 indicated that discrete silicalite nanocrystals with irregular disk-like morphology were present in samples taken up to 4 h after the start of the hydrothermal reaction (Fig. 3a). The assembly of intact chains occurred between 4 and 6 hours after the start of hydrothermal treatment, and was associated with transformation of the precursor particles into well-defined pseudo-hexagonal prismatic tablets (Fig. 3b). Once formed, the concentration of chains, as well as the number of nanocrystals per chain did not significantly increase with time (Fig. 3c).
![(a) TEM image showing localized region of a silicalite nanoparticle chain with co-aligned crystals (bar = 100 nm). (b) Corresponding “single-crystal” electron diffraction pattern viewed along the [100] zone axis. Reflections for (040), (051), (060), (080)
(d-spacings, 4.95, 3.80, 3.31, 2.48 Å, respectively) are highlighted (arrows). The (051) reflection is 16.5° to the b* axis, and the chain direction lies parallel to the crystallographic b direction.](/image/article/2005/JM/b415155k/b415155k-f2.gif) | ||
| Fig. 2 (a) TEM image showing localized region of a silicalite nanoparticle chain with co-aligned crystals (bar = 100 nm). (b) Corresponding “single-crystal” electron diffraction pattern viewed along the [100] zone axis. Reflections for (040), (051), (060), (080) (d-spacings, 4.95, 3.80, 3.31, 2.48 Å, respectively) are highlighted (arrows). The (051) reflection is 16.5° to the b* axis, and the chain direction lies parallel to the crystallographic b direction. | ||
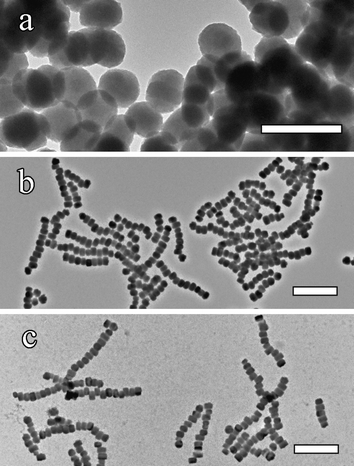 | ||
| Fig. 3 TEM images of samples taken after different hydrothermal periods: (a) 4 hr, showing disk-like silicalite particles and no chains; (b) 6 h, with initial chain formation, and (c) 15 h, mature chains. Scale bars (a) 500 nm; (b) and (c) 1 µm. | ||
Lowering the hydrothermal temperature from 150 °C to 120 °C for 24 hours in the presence of PDAC at a TEOS : PDAC molar ratio of 1 : 1.5 × 10−6 gave loosely aggregated spheroidal silicalite particles. No chain-like structures were observed. On the other hand, hydrothermal treatment under the same conditions but at 180 °C for 24 h resulted in an increase in the yield of morphologically well-defined silicalite crystals from 65% at 150 °C to 95%, with the consequence that disordered aggregates were formed by rapid agglomeration due to the high particle concentration. However, reducing the hydrothermal treatment time at 180 °C to 2 h lowered the yield to 60% and produced increased numbers of partially aligned chains of silicalite nanoparticles. Syntheses were also undertaken at 150 °C but in the presence of other polyelectrolytes, such as high molecular mass PDAC (Mr = 4 × 105, TEOS : PDAC = 1 : 0.5 × 10−6), polystyrene sulfate (PSS, Mr = 7 × 104, TEOS : PSS = 1 : 2.8 × 10−6) or polyethylene oxide (PEO, Mr = 8 × 103, TEOS : PEO = 1 : 2.5 × 10−5). In each case, chain superstructures were not observed.
The results indicate that stacking of silicalite nanoparticles into linear chains can occur spontaneously in the presence of PDAC when crystals of uniform size and shape are produced at moderate concentrations. The expression of well-defined (010) faces clearly facilitates organized stacking of the particles, provided that the particle concentration is not so high that disordered aggregates are produced by high rates of agglomeration. Chain formation was dependent on binding of the cationic polyelectrolyte to the negatively charged silicalite nanoparticles. Significantly, zeta potential measurements showed a charge reversal from a value of −25 mV for discrete silicalite particles formed in the absence of PDAC to +35 mV for the chain superstructures. No silicalite chains were produced under standard conditions when the polymer concentration was reduced beyond a critical level (TEOS : PDAC = 1 : 1.5 × 10−7) even though well-defined nanoparticles were formed, presumably because the zeta potential remained negative (−6.8 mV). In addition, relatively high amounts of PDAC (TEOS : PDAC = 7.7 × 10−6) inhibited silicalite crystallization due to polyelectrolyte-induced sequestration of the silicate reaction precursors. However, addition of PDAC to a preformed suspension of prismatic silicalite nanoparticles, followed by heating at 150 °C did not produce any chain superstructures although the zeta potential was highly positive (+55 mV), indicating that interlinking of the crystals was dependent on polymer–silicalite interactions associated with crystal growth rather than colloidal aggregation. One possibility is that adsorption of soluble PDAC/silicate hybrid conjugates and clusters onto the developing silicate (010) crystal surfaces not only reverses the surface charge but also presents reactive species for in situ silanol-mediated cross-linking of the particles during synthesis to produce crystallographically aligned arrays that are stable to sonication and thermal treatment.
Acknowledgements
We thank Asahi Kasei Corp. for financial support.Notes and references
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- G. A. Ozin, A. Kuperman and A. Stein, Angew. Chem., Int. Ed. Engl., 1989, 28, 359 CrossRef.
- S. A. Davis, M. Breulmann, K. H. Rhodes, B.-J. Zhang and S. Mann, Chem. Mater., 2001, 13, 3218 CrossRef CAS.
- K. H. Rhodes, S. A. Davis, F. Xaruso, B.-J. Zhang and S. Mann, Chem. Mater., 2000, 12, 2832 CrossRef CAS; X. D. Wang, W. L. Yang, Y. Tang, Y. J. Wang, S. K. Fu and Z. Gao, Chem. Commun., 2000, 2161 RSC; A. G. Dong, Y. J. Wang, Y. Tang, N. Ren, Y. H. Zhang and Z. Gao, Chem. Mater., 2002, 14, 3217 CrossRef CAS; A. G. Dong, N. Ren, W. L. Yang, Y. J. Wang, Y. H. Zhang, D. J. Wang, H. H. Hu, Z. Gao and Y. Tang, Adv. Funct. Mater., 2003, 13, 943 CrossRef CAS.
- B.-J. Zhang, S. A. Davis, N. H. Mendelson and S. Mann, Chem. Commun., 2000, 781 RSC; S. M. Huang, J. Am. Chem. Soc., 2000, 122, 3530 CrossRef CAS.
- B.-J. Zhang, S. A. Davis and S. Mann, Chem. Mater., 2002, 14, 1369 CrossRef CAS.
- L. Huerta, C. Guillem, J. Latorre, A. Beltran, D. Beltran and P. Amoros, Chem. Commun., 2003, 1448 RSC.
- K. Ha, Y.-J. Lee, Y. S. Chun, Y. S. Park, G. S. Lee and K. B. Yoon, Adv. Mater., 2001, 13, 594 CrossRef CAS; K. Ha, Y.-J. Lee, D.-Y. Jung, J. H. Lee and K. B. Yoon, Adv. Mater., 2000, 12, 1614 CrossRef CAS.
- A. Kulak, Y. J. Lee, Y. S. Park, H. S. Kim, G. S. Lee and K. B. Yoon, Adv. Mater., 2002, 14, 526 CrossRef CAS.
- D. Walsh, A. Kulak, K. Aoki, T. Ikoma, J. Tanaka and S. Mann, Angew. Chem., Int. Ed., 2005 Search PubMed , in press.
- S. P. B. Kremer, C. E. A. Kirschhock, A. Aerts, K. Villani, J. A. Martens, O. I. Lebedev and G. V. Tendeloo, Adv. Mater., 2003, 15, 1705 CrossRef CAS.
- Typically, 16.00 g of TEOS was slowly added to a stirred aqueous solution containing 27.32 g of 1 M TPAOH and 10 g of 20 wt% PDAC (Mw = 1–2 × 105; TEOS : PDAC molar ratio = 1 : 1.5 × 10−6). After stirring for 24 hours at room temperature, large agglomerates were removed, and 10 mL of the resulting cloudy suspension poured into a 20 mL hydrothermal bomb, and placed in a preheated oven at 150 °C and left at this temperature for 24 hours. After cooling to room temperature, the white suspension was centrifuged and the supernatant fluid removed, and deionised water added and the precipitate resuspended by sonication. This washing procedure was repeated several times to give a suspension 6–7 wt% in solids that slowly sedimented over a period of a few days.
- XRD d-spacings (nm): 1.11 (101)/(011), 1.0 (200), (020), 0.97 (111), 0.67 (300), 0.64 (012), 0.60 (301), 0.57 (131), 0.38 (501)/(051), 0.37 (033)/(303), 0.36 (133). Mean particle sizes (TEM); TEOS : PDAC = 1 : 1.5 × 10−6, width = 245 nm, thickness = 140 nm; TEOS : PDAC = 1 : 0.3 × 10−6, width = 200 nm, thickness = 120 nm. Mean number of crystals per chain = 5.5.
- FTIR absorption bands (cm−1): 450 (Si–O def.), 795, 1091, 1217 (Si–O str.), 970 (Si–OH str.), 1389, 1457, 1471 (C–CH3 def.), 2879, 2941, 2974 (C–CH3 str.). The absorption at ca. 450 cm−1 indicates the existence of SiO2 double chain bonding characteristic of the silicalite structure. TG analysis for control and PDAC-added samples showed in both cases ca.18 wt% total loss up to 1000 °C and ca.12 wt% loss between 280 and 450 °C.
- Samples were heated at a rate of 2 °C min−1 to 550 °C and kept at this temperature for 20 hours, followed by cooling to room temperature and sonication-induced redispersion in ethanol.
- Time dependent studies were carried out at TEOS : PDAC = 1 : 1.5 × 10−6 using the above synthesis procedure. The hydrothermal bomb was removed from the oven after 1, 2, 4, 6, 15, 28, or 45 hours from the start of the hydrothermal treatment. After cooling to room temperature, the white suspension was diluted with water and investigated by TEM and SEM. The yields were recorded after washing and drying. Percentage yields of silicalite nanoparticles were calculated with respect to the weight of silica in the starting solutions.
| This journal is © The Royal Society of Chemistry 2005 |
