Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces
Filip
Petersson
a,
Andreas
Nilsson
a,
Cecilia
Holm
b,
Henrik
Jönsson
c and
Thomas
Laurell
*a
aDepartment of Electrical Measurements, Lund Institute of Technology, P.O. Box 118, S-221 00 Lund, Sweden. E-mail: thomas.laurell@elmat.lth.se; Fax: +46 46 222 45 27
bSection for Molecular Signalling, Department of Cell and Molecular Biology, Lund University, Lund, Sweden
cDepartment of Cardiothoracic Surgery, Lund University Hospital, Lund, Sweden
First published on 17th September 2004
Abstract
Improved continuous acoustic particle separation (separation efficiency close to 100%) and separation of erythrocytes (red blood cells) from lipid microemboli in whole blood is reported.
Introduction
It is a well-known fact that particles in fluid suspensions may be enriched at defined positions by means of forces generated by acoustic standing wave fields. Theoretical pioneers in this discipline were King,1 Gorkov,2 and Yosioka and Kawasima.3 Their acoustic force theories have since been used by several groups of researchers in particle separation applications.4–9 A new approach to continuous separation of particles in microfluidic channels was recently proposed by Nilsson et al.,4 enriching particles in the pressure nodes of an ultrasonic standing wave in a continuously perfused microchannel. Earlier version of the system4 reported particle enrichment in a double pressure node configuration, collecting enriched particles in the side outlets of a triple channel outlet. The current set-up described in this communication demonstrates improved particle separation efficiency, defined as the fraction of particles collected in the centre outlet. Also, successful separation of particles having different physical properties is reported, i.e. lipid vesicles were separated from erythrocytes. These accomplishments were attributed to the further miniaturisation of the separation device to a channel width of 350 µm, a depth of 125 µm and operation in a half wave length resonance mode, providing higher acoustic forces on the particles.During cardiac surgery supported by a heart-lung machine a massive embolization of lipid particles occur in the brain when shed blood is returned to a patient via a filter.10 The lipid particles are derived from triglycerides leaking from fat cells during surgery in adipose tissue. The embolization is associated with cognitive dysfunction observed after surgery.11 The techniques currently available for blood wash do not meet the demand to remove these lipid particles. The most common method to wash blood is based on centrifuges which are burdened with a number of drawbacks, i.e. they only handle larger amounts of blood (≈ 0.5 l), are harmful for the blood cells,12 need specially trained personnel, are not continuous and display a limited lipid particle elimination.
The technology presented in this communication offers a solution to the embolization problem by employing the possibility of discriminating erythrocytes from lipid particles. In addition, when fully developed and implemented clinically, it reduces the demand for allogenic blood and reduces or eliminates blood transfusion related incompatibilities. The primary acoustic radiation force equation13 tells us that the acoustic force can move particles either towards a node or an anti-node of a standing wave depending on their densities and compressibilities. If the particles are red blood cells and lipid droplets in blood plasma, the erythrocytes gather in the pressure node (in the centre of the channel) while the lipid particles gather in the pressure anti-nodes (by the side walls), Fig. 1. At the end of the channel the red blood cells exit through the centre outlet while the lipid particles exit through the side outlets, separating the two particle types, Fig. 2.
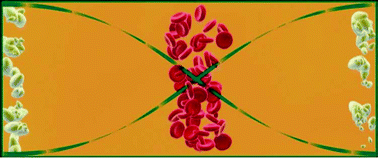 | ||
| Fig. 1 Cross-section of channel with erythrocytes and lipid particles. When the ultrasound is turned on the two particle types are separated. | ||
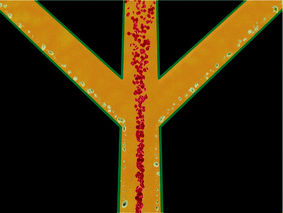 | ||
| Fig. 2 If the main channel is split into three outlet channels the laminar flow properties makes it possible to collect erythrocytes and lipid particles separately. | ||
Experiments and results
The experiments were performed as reported in ref. 4 with the modification that a half wavelength standing wave was used. In vitro experiments were performed on a particle suspension composed of a 2% concentration of 5 µm polyamide spheres (blood phantom), see ref. 4 for details.All separations were performed at low Reynolds numbers, (Re < 40). Considerably improved separation efficiencies (close to 100% of the particles were collected in 1/3 of the original fluid volume, Fig. 3) as compared to earlier data were accomplished at lower actuation voltages (12 Vpp), and at a four times higher flow speed.
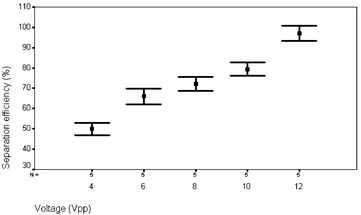 | ||
| Fig. 3 Separation efficiency of the 350 µm separation channel versus applied voltage to the piezo ceramic element. Flow rate 0.3 ml min−1. | ||
To evaluate the systems ability to separate lipid particles from red blood cells a mixture of bovine blood, saline solution and a phospholipid-stabilized emulsion of triolein was used. The triolein emulsion was prepared according to ref. 14 with some modifications. A total amount of 320 mg triolein, tritium labelled and unlabelled, and 3.2 mg of phospholipids were sonicated in 7.2 ml of PBS. Following sonication, 0.8 ml of 20% BSA in PBS was added. The lipid emulsions were always used within 5 h from the time of preparation. The lipid content was measured by a scintillation counter (Wallac Guardian 1414 Liquid Scintillation Counter, PerkinElmer Life and Analytical Sciences Inc., Boston, MA, USA), according to standard scintillation counting protocol,15 with the modification of using Ultima Gold (Packard Biosciences, Boston, MA, USA) as scintillation liquid. The lipid separation efficiency was defined as the fraction of lipid particles exiting through the side outlets.
More than 70% of the erythrocytes were collected in 1/3 of the original fluid volume and more than 80% of the lipid particles were removed, Figs. 4 and 5. The applied voltage was 10 Vpp, the flow rate 0.3 ml min−1, the concentration of red blood cells 2.5% and the concentration of lipid particles 1%. The fraction of lipid particles removed seemed to be independent of the concentration of erythrocytes. It was not possible to quantify the systems ability to remove human lipids in human blood collected during cardiac surgery since no reliable method was available. However, visual observations of the separation process performed on human blood could be made, displaying steady streams of lipid particles flowing along the side walls and a well defined band of red blood cells exiting the system through the centre outlet channel, Fig. 6.
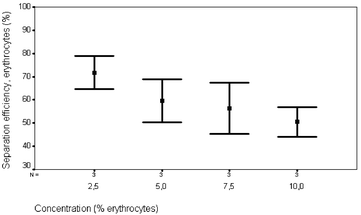 | ||
| Fig. 4 Separation efficiency versus erythrocyte concentration. The total flow rate was 0.3 ml min−1 and a voltage of 10 Vpp was applied to the piezo ceramic element. | ||
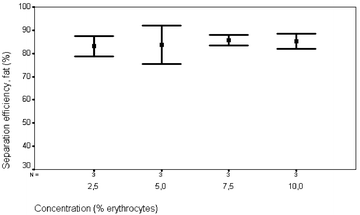 | ||
| Fig. 5 Separation efficiency of lipid particles versus erythrocyte concentration. Flow rate 0.3 ml min−1, actuation voltage 10 Vpp and input lipid concentration 1%. | ||
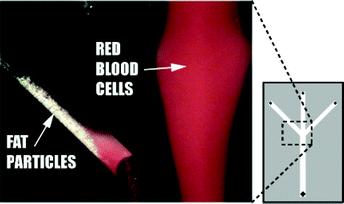 | ||
| Fig. 6 Human lipid particles separated from human erythrocytes at the trifurcation of 350 µm separation chip with ultrasound turned on. | ||
The degree of hemolysis, red blood cell lysis, was measured using a HemoCue Plasma/Low Hb meter (HemoCue AB, Ängelholm, Sweden). No change in the degree of hemolysis was detected after the separation process compared to before, indicating that the process was harmless to the red blood cells.
Conclusion
Separation efficiencies close to 100% could be reached for polyamide spheres using the new 350 micrometer separation device. Separation efficiencies of above 80% for triglyceride emulsions were obtained. Separation of micro emboli from human erythrocytes collected during surgery was visually confirmed. No hemolysis induced by the separation process was detected. To accomplish a system for full scale clinical use 100–200 separation channels have to be used in parallel to reach a reasonably high throughput.Acknowledgments
The authors wish to thank The Swedish Research Council for their financial support. The authors also wish to thank Ellco Food AB (Kävlinge, Sweden) for their kind donations of bovine blood.References
- L. V. King, On the acoustic radiation pressure on spheres, Proc. Roy. Soc. Lond., 1934, A147, 212–240 Search PubMed
.
- L. P. Gorkov, On the forces acting on a small particle in an acoustic filed in an ideal fluid, Sov. Phys. Doklady, 1962, 6(9), 773–775 Search PubMed
.
- K. Yosioka and Y. Kawasima, Acoustic radiation pressure on a compressible sphere, Acustica, 1955, 5167–173 Search PubMed
.
- A. Nilsson, F. Petersson, H. Jönsson and T. Laurell, Acoustic control of suspended particles in micro fluidic chips, Lab Chip, 2004, 4, 131–135 RSC
.
- M. Gröschl, Ultrasonic separation of suspended particles—Part I: Fundamentals, Acust. Acta Acust., 1998, 84, 432–447 Search PubMed
.
- J. J. Hawkes and W. T. Coakley, Force field particle filter, combining ultrasound standing waves and laminar flow, Sens. Actuators, B, 2001, 75, 213–222 CrossRef
.
- D. A. Johnson and D. L. Feke, Methodology for fractionating suspended particles using ultrasonic standing wave and divided flow fields, Sep. Tech., 1995, 5, 251–258 CrossRef CAS
.
- K. Yasuda, S. Umemura and K. Takeda, Concentration and fractionation of small particles in liquid by ultrasound, Jpn. J. Appl. Phys. 1, 1995, 34:5B, 2715–2720 CrossRef
.
- N. R. Harris, M. Hill, S. Beeby, Y. Shen, N. M. White, J. J. Hawkes and W. T. Coakley, A silicon microfluidic ultrasonic separator, Sens. Actuators, B, 2003, 95, 425–434 CrossRef
.
- D. M. Moody, W. R. Brown, V. R. Challa, D. A. Stump, D. M. Reboussin and C. Legault, Brain microemboli during cardiac surgery or aortography, Ann. Neurol., 1990, 28(4), 477–486 CAS
.
- E. P. Mahanna, J. A. Blumenthal, W. D. White, N. D. Croughwell, C. P. Clancy, L. R. Smith and M. F. Newman, Defining neuropsychological dysfunction after coronary bypass grafting, Ann. Thorac. Surg., 1996, 61(5), 1342–1347 CrossRef CAS
.
- C. T. Klodell, J. D. Richardson, T. M. Berdamini and D. A. Spain, Does cell-saver blood administration and free hemoglobin load cause renal dysfunction?, Am. Surg., 2001, 67(1), 44–47 Search PubMed
.
-
W. L. Nyborg, Physical principles of ultrasound, in Ultrasound: Its Applications in Medicine and Biology, ed. F. J. Fry, Elsevier, New York, 1978, Pt. 1, pp. 1–76 Search PubMed
.
-
C. Holm and T. Österlund, Hormone-sensitive lipase and neutral cholesteryl ester lipase, Methods in Molecular Biology: Lipase and Phospholipase Protocols, ed. M. H. Doolittle and K. Reue, Humana Press Inc., Totwa, NJ, 1999, vol. 109 Search PubMed
.
- S. Evangelista, P. Cochet, N. Bromet, M. Criscuoli and C. A. Maggi, A distribution study with 14C-otilonium bromide in the rat: Evidence for selective tropism for large intestine after oral administration, Drug Metab. Dispos., 2000, 28, 643–647 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2005 |
