Single- and multi-photon excited fluorescence from serotonin complexed with β-cyclodextrin
Roger H.
Bisby
*a,
Stanley W.
Botchway
b,
Shakeela
Dad
a and
Anthony W.
Parker
b
aBiosciences Research Institute, University of Salford, Salford, UK M5 4WT. E-mail: r.h.bisby@salford.ac.uk; Fax: +44 161 295 5015; Tel: +44 161 295 4912
bLasers for Science Facility, Rutherford Appleton Laboratory, Chilton, UK OX11 0QX
First published on 11th November 2005
Abstract
The fluorescence of serotonin on binding with β-cyclodextrin has been studied using both steady state and time-resolved methods. Steady state fluorescence intensity of serotonin at 340 nm showed ∼30% increase in intensity on binding with KA ∼ 60 dm3 mol−1 and the fluorescence lifetimes showed a corresponding increase. In contrast, the characteristic green fluorescence (‘hyperluminescence’) of serotonin observed upon multiphoton near-infrared excitation with sub-picosecond pulses was resolved into two lifetime components assigned to free and bound serotonin. The results are of interest in relation to selective imaging and detection of serotonin using the unusual hyperluminescence emission and in respect to recent determinations of serotonin by capillary electrophoresis in the presence of cyclodextrin. The results also suggest that hyperluminescence occurs from multiphoton excitation of a single isolated serotonin molecule.
Introduction
Levels of the neurotransmitter serotonin have been linked to clinical depression and suicide.1 This has consequently created considerable interest in the development of analytical methods for determining serotonin levels in biological samples such as cerebrospinal fluid.2 A recent report describes the use of capillary electrophoresis in combination with hydroxypropyl-β-cyclodextrin for analyzing serotonin levels in brain microdialysis.3 The importance of neurotransmitters has also stimulated research to develop methods to locate them within living cells4 at physiological levels, in particular, and non-linear spectroscopic methods linked to microscopy have much potential in the field of neurobiology.5 The peak UV absorption of the 5-hydroxyindole chromophore in both 5-hydroxytryptophan and serotonin occurs at a slightly longer wavelength than tryptophan. This has enabled the use of 5-hydroxytryptophan as a fluorescence probe to study conformation and mobility within proteins.6 In RBL rat mast cells, confocal three-photon excitation using ultrafast near-infrared laser pulses has enabled imaging of serotonin distribution and release by monitoring the UV fluorescence at 340 nm.7,8 We and others are presently exploring the further possibility for selective intracellular imaging of serotonin by taking advantage of the specific green emission (‘hyperluminescence’) produced by multiphoton excitation of serotonin and 5-hydroxytryptophan.9,10 However, the identity of the molecular photoproduct responsible for hyperluminescence remains elusive. The supra-linear dependence of hyperluminescence intensity on serotonin concentration in phosphate buffer11 implies the possible involvement of a dimeric or higher aggregate photoproduct, although rapid capillary electrophoresis experiments indicate the photoproduct is of a similar size to serotonin itself.12 The present experiments, in which a molecule of serotonin is isolated within a cyclodextrin cavity, seek to resolve some aspects of this fundamental issue.Cyclodextrins, especially β-cyclodextrin (cycloheptaamylose), have found varied applications including drug solubilisation and delivery and in analytical chemistry procedures.13 This wide range of applications comes from their ability to form inclusion complexes and act as a host for various guest molecular species. Cyclodextrins possess a relatively hydrophobic cavity with the more polar hydroxyl groups arranged around the external rim. These features provide both isolation of the guest molecule from the surrounding environment as well as a means of improving aqueous solubility of hydrophobic compounds. Cyclodextrins are able to modify the behaviour of electronically excited states of organic molecules,14 for example in permitting room temperature phosphorescence,15 enhancement of fluorescence quantum yields16–19 and influencing the outcome of photochemical reactions.20 This present work has sought to further the earlier work using capillary electrophoresis in combination with hydroxypropyl-β-cyclodextrin3 and quantify the nature of the serotonin:β-cyclodextrin complex and characterise the photophysical properties of both UV fluorescent and multiphoton hyperluminescence from serotonin.
Experimental
All chemicals including serotonin and β-cyclodextrin were purchased from Sigma-Aldrich. Solutions were prepared in phosphate buffer (20 mmol dm−3, pH 7.0) containing NaCl (0.15 mol dm−3).Fluorescence spectra were measured using a Spex Fluoromax spectrofluorimeter using the manufacturer's correction curve to obtain corrected spectra. Fluorescence lifetimes were measured using the time-correlated single-photon counting technique using as the excitation source a Spectra-Physics Ti:sapphire laser (120 fs pulses, 80 MHz) pumped by a 20 W argon ion laser (Spectra Physics Ltd). For one-photon excitation at 300 nm, the Ti:sapphire laser output was shifted to 600 nm in a Mira OPO (Coherent) and doubled to 300 nm using a type 1 β-barium borate (BBO) crystal, delivering ∼4 mW to the sample. Fluorescence at 340 nm from a sample in a standard 1 cm quartz cuvette was selected by a monochromator (M500, IBH) and detected with a Becker & Hickl GmbH photon-counting photomultiplier (Model PMH-100) linked to a Becker and Hickel SPC700 multichannel analyser card. Fluorescence decays were deconvoluted with the instrument response function using Edinburgh Instruments software. Multiphoton excitation at 750 nm used the direct output of the Ti:sapphire laser (∼60 mW at the sample). A drop of the sample on a cover slip was placed on the stage of a fluorescence microscope (Nikon TE2000) and green emission selected with a 505 nm interference filter (500IU25, Comar, UK). The analysis of binding data by non-linear least squares analysis used the Grafit computer program.
Results and discussion
Ultraviolet fluorescence
Serotonin fluoresces in aqueous solution at pH 7 with a maximum at 334 nm on excitation at 300 nm. Addition of β-cyclodextrin to solutions of serotonin (5 μmol dm−3) in solutions buffered with phosphate (20 mmol dm−3, pH 7.0) and NaCl (0.15 mol dm−3) resulted in a small enhancement of the fluorescence intensity (Fig. 1). Neither the excitation nor emission spectra showed any significant changes in shape or peak wavelengths with the addition of β-cyclodextrin. This is consistent with the observation that the fluorescence properties of 5-hydroxyindole are relatively insensitive to solvent polarity.21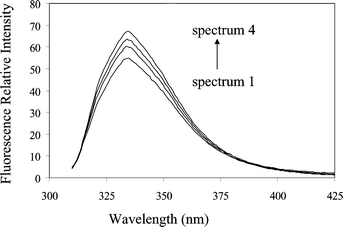 | ||
| Fig. 1 Fluorescence spectra from serotonin (5 μmol dm−3) excited at 300 nm in solutions at pH 7 containing: 0 (curve 1); 5 (curve 2); 10 (curve 3) and 20 (curve 4) mmol dm−3 β-cyclodextrin. | ||
The binding parameters for the serotonin (S)–β-cyclodextrin (β-CD) complex were evaluated on the basis of formation of a 1 : 1 complex as has previously been established in similar cases:16,17
| S + β-CD ⇌ [S–β-CD] | (1) |
The association constant of the complex, KA, was found by direct non-linear least squares fitting of plots of fluorescence intensity (F) versus β-cyclodextrin concentration according to the binding eqn (2), where F0 is the intensity in the absence of β-cyclodextrin and Fmax is the maximal increase in intensity at infinite β-cyclodextrin concentration:
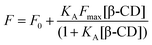 | (2) |
Intensities were obtained by integration of the fluorescence spectra on excitation at 300 nm and are shown plotted against β-cyclodextrin concentration in Fig. 2. The best fit to eqn (2) is shown by the line with KA = 53.2 ± 17.3 dm3 mol−1 and the value of Fmax corresponding to 33.8 ± 6.1% increase in fluorescence intensity of the fully complexed serotonin. (Note: the precision of the measurements of such a small KA for serotonin:β-cyclodextrin binding is limited by the solubility of β-cyclodextrin and the comparatively small change in fluorescence intensity observed.) It is noteworthy that the value of KA for binding of serotonin by β-cyclodextrin obtained here is less than those for tryptamine17 and tryptophan16 (160 ± 30 and 184 ± 18 dm3 mol−1 respectively) but the overall values for increases in fluorescence intensity on binding are similar.
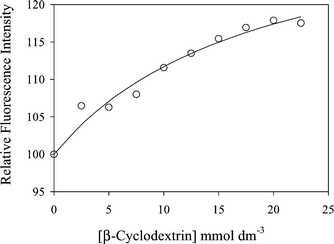 | ||
| Fig. 2 Increase in fluorescence intensity from solutions of serotonin (5 μmol dm−3) at pH 7 on addition of β-cyclodextrin. The solid line indicates the fit with KA = 53.2 dm3 mol−1, as described in the text. | ||
Time-resolved fluorescence decays of serotonin, excited at 300 nm and detected at 340 nm, were measured using the time-correlated single-photon counting technique. In the absence of cyclodextrin, the fluorescence decay was satisfactorily deconvoluted (χ2 < 1.2) as a single exponential with a lifetime of 3.65 ns, in good agreement with a previous determination of 3.80 ns at pH 7.22 Addition of β-cyclodextrin resulted in an increase in the fluorescence lifetime (Fig. 4), reaching a value of 4.24 ns in the presence of 20 mmol dm−3 β-cyclodextrin. The increase in fluorescence lifetime (16%) closely matches the rise of steady state fluorescence intensity (18%) at 20 mmol dm−3 β-cyclodextrin recorded above.
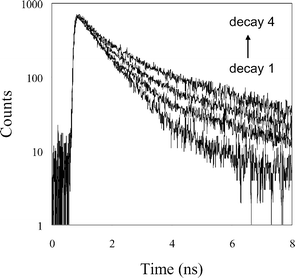 | ||
| Fig. 3 Nanosecond time-resolved fluorescence decays at 505 nm following multiphoton excitation at 750 nm of serotonin (1 mmol dm−3) in solutions (pH 7) containing β-cyclodextrin: 0 (trace 1); 5 (trace 2); 10 (trace 3) and 20 (trace 4) mmol dm−3. | ||
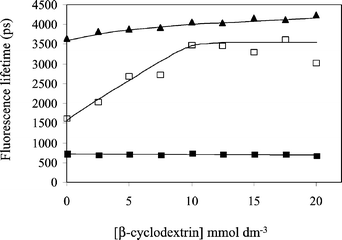 | ||
| Fig. 4 Fluorescence (hyperluminescence) lifetimes of the biexponential decay from serotonin (1 mmol dm−3) on multiphoton excitation at 750 nm and pH 7 with increasing β-cyclodextrin concentration. Emission was observed at 505 nm. Increasing the β-cyclodextrin concentration had no effect on the short lifetime component (■), whilst the lifetime of longer component increased (□). Also shown are the single exponential lifetimes of the UV fluorescence at 340 nm, excited at 300 nm (▲). | ||
Time-resolved hyperluminescence
Multiphoton excitation of serotonin using an ultrafast near-infrared laser is known to produce the green fluorescence at 505 nm (hyperluminescence). This corresponds to a six-photon process, involving the initial formation of photochemical intermediate in a four-photon step, followed by excitation of the intermediate by a further two photons.9 For this work we used a focussed laser beam (750 nm, 120 fs pulses, 80 MHz, 56 mW at the sample) produced by a Ti:sapphire laser. The decay of hyperluminescence from solutions of serotonin (1 mmol dm−3, pH 7.0) was biexponential. Due to the inherently weaker emission in the hyperluminescence experiments, a higher serotonin concentration (1 mmol dm−3) was used. However over most of the range of cyclodextrin concentrations used (2.5 to 20 mmol dm−3), the cyclodextrin is present in modest excess (≥ 5 : 1 molar ratio) and analysis of data using eqn (2) is justified. Representative time-resolved decays are shown in Fig. 3 and analysis shows they contain a short lifetime (τ1) of 710 ± 17 ps that is found to be independent of cyclodextrin concentration. However, the lifetime of a second component (τ2) increased from 1.65 ns in the absence of β-cyclodextrin to a limiting value of 3.4 ± 0.3 ns above β-cyclodextrin concentrations of 10 mmol dm−3 (Fig. 4). A more complex analysis of the data was not attempted and the increase in the long lifetime component to this limiting value is taken to indicate the eventual predominance of the longer lifetime component due to serotonin binding within the β-cyclodextrin cavity. The fractional intensity of the longer lifetime component (f2), defined by eqn (3) where α1 and α2 are the pre-exponential factors from the fitting analysis,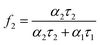 | (3) |
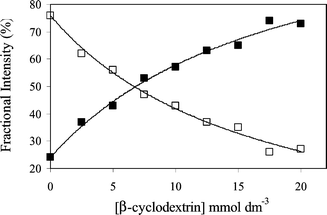 | ||
| Fig. 5 Intensity contributions in the biexponential decay of serotonin fluorescence (hyperluminescence) at 505 nm after multiphoton excitation at 750 nm with increasing β-cyclodextrin concentration. Relative intensity (percentage) contributions are shown for the short lifetime component (□) and the long lifetime component (■). | ||
As similarly reported in a study of fluorescence lifetimes measured on the binding 2-amino-5,6-dimethylbenzimidazole to β-cyclodextrin,19 the hyperluminescence decays from serotonin clearly contain exponential components attributable to bound and free species. For the hyperluminescence the principal lifetimes of bound and free serotonin are 3.4 ns and 0.71 ns respectively. It might have been anticipated that differentiation of bound and free serotonin would be observed in the single-photon-excited ultraviolet (340 nm) fluorescence decays from serotonin. However since both intensity and lifetimes show equivalent small increases of only 16–18% over the available β-cyclodextrin concentration range, the two lifetimes may not have been identified within the deconvolution process. Hyperluminescence from 5-hydroxytryptophan has been shown to have a similarly short lifetime in aqueous solution of 0.91 ns.10 Unlike the fluorescence lifetime of the normal ultraviolet (340 nm) fluorescence of 5-hydroxytryptophan,21 hyperluminescence lifetimes were found to be very responsive to solvent,10 increasing up to 3.1 ns in 90% ethanol in water, a value rather similar to the lifetime identified here with the hyperluminescent species bound within the β-cyclodextrin cavity.
Hyperluminescence of molecules such as serotonin and 5-hydroxytryptophan offers new opportunities in the detection11 and imaging23 of these compounds. The transient species responsible for hyperluminescence remains unidentified, although new routes to multiphoton excitation of serotonin have been explored recently.24 Capillary electrophoresis experiments have been used to eliminate dimer or higher multimers of serotonin as being responsible for the intermediate that produces the hyperluminescence.12 In biological systems there is the possibility that serotonin may be bound to proteins such as albumin, and that binding may affect attempts to quantify or image serotonin using hyperluminescence. The results show that whilst native ultraviolet fluorescence may be relatively unaffected by binding, the hyperluminescence lifetime is more sensitive and might be used as an indicator of such interactions.
In the present experiments there are two possibilities leading to hyperluminescence from serotonin with the lifetime of 3.4 ns. This value is characteristic of the fluorophore being located within an environment with a polarity lower than water. Thus the first possibility is the photochemical conversion of serotonin to the hyperluminescent intermediate arises from the four-photon excitation (at 750–830 nm) of a serotonin molecule already complexed within the β-cyclodextrin cavity. This intermediate whilst still complexed may then be further excited by a further two photons to generate the hyperluminescence. In this case the hyperluminescence comes from an isolated single serotonin molecule. An alternative mechanism for the hyperluminescence with 3.4 ns lifetime is that the initial intermediate is formed by the four-photon process in solution. This may then associate with cyclodextrin during its lifetime (>100 μs)9 before being excited to give hyperluminescence from within the cyclodextrin cavity. In the latter case the similarity of the association constants in the static fluorescence observed at 340 nm generated by UV excitation and the time-resolved hyperluminescence experiments indicate similar sizes and polarities of the serotonin molecule and the intermediate species. These arguments support the hypothesis that hyperluminescence occurs from a monomer species derived from serotonin, and that it should be possible to observe hyperluminescence from a single residue of 5-hydroxytryptophan within a protein.
Abbreviations
UV—ultraviolet; RBL—rat basophilic leukaemia.Acknowledgements
The authors thank the Biological and Biotechnological Research Council (BBRSC) for a grant in support of this work, CCLRC for access to the Central Laser Facility and Mathew Dillingham for technical assistance.References
- A. S. Elhwuegi, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2004, 28, 435–451 CrossRef CAS.
- M. Asberg, Ann. N. Y. Acad. Sci., 1997, 836, 158–181 CrossRef CAS.
- N. Beturquia, F. Couderc, V. Sauvinet, C. Ordset, S. Parrot, C. Bayle, B. Renaus and L. Denoroy, Electrophoresis, 2005, 26, 1071–1079 CrossRef.
- S. Okumoto, L. L. Looger, K. D. Michever, R. J. Reimer, S. J. Smith and W. B. Frommer, Proc. Natl. Acad. Sci. USA, 2005, 102, 8740–8745 CrossRef CAS.
- J. Merz, Curr. Opin. Neurobiol., 2004, 14, 610–616 CrossRef.
- J. B. A. Ross, A. G. Szabo and C. W. V. Hogue, Methods Enzymol., 1997, 278, 151–190 CAS.
- S. Maiti, J. B. Shear, R. M. Williams, W. R. Zipfel and W. W. Webb, Science, 1997, 275, 530–532 CrossRef CAS.
- R. M. Williams, J. B. Shear, W. R. Zipfel, S. Maiti and W. W. Webb, Biophys. J., 1999, 76, 1835–1846 CrossRef CAS.
- J. B. Shear, C. Xu and W. W. Webb, Photochem. Photobiol., 1997, 65, 931–936 CrossRef CAS.
- R. H. Bisby, M. Arvanitidis, S. W. Botchway, I. P. Clark, A. W. Parker and D. Tobin, Photochem. Photobiol. Sci., 2003, 2, 157–162 RSC.
- M. L. Gostkowski, J. Wei and J. B. Shear, Anal. Biochem., 1998, 260, 244–250 CrossRef CAS.
- M. J. Gordon, E. Okerberg, M. L. Gostkowski and J. B. Shear, J. Am. Chem. Soc., 2001, 123, 10780–10781 CrossRef CAS.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1753 CrossRef CAS.
- P. Bortolus and S. Monti, Adv. Photochem., 1996, 21, 1–119 Search PubMed.
- S. Scypinski and L. J. C. Love, Anal. Chem., 1984, 56, 331–336 CrossRef CAS.
- A. Orstan and J. B. A. Ross, J. Phys. Chem., 1987, 91, 2739–2745 CrossRef CAS.
- R. E. Galian, A. V. Veglia and R. H. de Rossi, Analyst, 1998, 123, 1587–1591 RSC.
- C. Donze, E. Rizzarelli and G. Vecchio, Supramol. Chem., 1998, 10, 33–42 CrossRef CAS.
- M. A. El-Kemary and I. M. El-Mehasseb, Talanta, 2004, 62, 317–322 CrossRef CAS.
- K. S. S. P. Rao, S. M. Hubig, J. N. Moorthy and J. K. Kochi, J. Org. Chem., 1999, 64, 8098–8104 CrossRef CAS.
- K. Lotte, R. Plessow and A. Brockhinke, Photochem. Photobiol. Sci., 2004, 3, 348–359 RSC.
- A. Chattopadhyay, R. Rukmini and S. Mukerjee, Biophys. J., 1996, 71, 1952–1960 CrossRef CAS.
- S. W. Botchway, I. Barba, R. Jordan, R. Harmston, P. M. Haggie, S. P. Williams, A. M. Fulton, A. W. Parker and K. M. Brindle, Biochem. J., 2005, 390, 787–790 CrossRef CAS.
- M. L. Gostkowski, R. Allen, M. L. Plenert, E. Okerberg, M. J. Gordon and J. B. Shear, Biophys. J., 2004, 86, 3223–3229 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2006 |
