An improved microbial fuel cell with laccase as the oxygen reduction catalyst
Olivier
Schaetzle
a,
Frédéric
Barrière
*a and
Uwe
Schröder
b
aUniversité de Rennes 1, CNRS UMR n° 6226, Sciences Chimiques de Rennes, Equipe MaCSE, France. E-mail: frederic.barriere@univ-rennes1.fr
bInstitut für Biochemie, Ernst-Moritz-Arndt Universität, Felix-Hausdorff-Straße 4, 17487, Greifswald, Germany
First published on 14th October 2008
Abstract
In this communication, we report the assembly and study of a biofuel fuel cell consisting of a microbial anode oxidizing organic substrates and an enzymatic cathode reducing oxygen.
Broader contextMicrobial fuel cells (MFCs), generate electrical power through the anodic oxidation of organic substrates mediated by micro-organisms, while the reduction of the electron acceptor (oxidant) occurs at the cathode. One of the proposed applications of such bio-electrochemical devices is the bio-remediation of wastewater coupled to the production of bio-energy. In order to increase the power output of these fuel cells, work is underway to improve the fuel cell design and the electrode reaction kinetics. At the cathode, oxidants such as potassium permanganate or ferricyanide have been used to boost MFCs power output. From a sustainability point of view however, molecular oxygen is the oxidant of choice since it is freely available and can be reduced to water. In this communication we have considered the linking of an efficient O2-reducing enzymatic cathode to a microbial anode. We found that this combination gives a higher maximum power output compared to the MFC based on ferricyanide at the cathode. We advocate that enzymatic cathodes should be considered to improve the performance of MFCs, but this must be weighed against the advantages/drawbacks of chemical and microbial alternatives for the catalysis of molecular oxygen reduction at the cathode. |
Microbial fuel cells (MFCs) are bioelectrochemical devices that harvest electricity from organic substrates.1 The principle of an MFC, shown in Fig. 1, rests on the oxidation of the fuel through microbial catabolism in the anaerobic anodic compartment of a fuel cell. The current is generated by diverting electrons from the microbe membrane respiratory chain to the anode.1,19 After flowing through an external load, the electrical current reduces the terminal electron acceptor, usually O2, at the cathode of the fuel cell. Electron transfer between a bacterium and an electrode is likely to occur through different mechanisms, as shown on Fig. 1, left, i.e. (A) direct electron transfer, (B) direct electron transfer through pili, (C) mediated electron transfer through naturally exported or artificially added redox mediators, and (D) anodic oxidation of excreted metabolites (e.g.formate).2,3,24 The large-scale and ambitious targeted application of MFCs is the coupling of bio-electricity production to wastewater treatment (removal of organic pollutants).4 Despite the impressive progress made in the study and optimization of MFCs in the last decade, the best reported power output of MFC prototypes (of the order of 100 Watt per cubic meter) still remains at least one order of magnitude too low for the implementation of an economical process. The factors limiting MFC power output are however well understood.5 Hence, work is underway towards better fuel cell design and the minimizing of overpotential at both electrodes, in order to make MFC technology more efficient and competitive. The oxidizer of choice at the cathode of MFCs is O2 since it is widely available and can be reduced down to innocuous H2O.30 Other candidates as terminal electron acceptors in MFCs include added species to the catholyte such as potassium ferricyanide or permanganate. The addition of these metal-based oxidizers is however expensive, not sustainable and not compatible with a continuous-flow system.1 In this context, it is important to improve the kinetics of the notoriously difficult cathodic oxygen reduction in order to contribute to more efficient and sustainable MFCs.6 Indeed, even at expensive platinum or other precious metals electrodes, the overpotential for oxygen reduction remains significant. Alternatives to the direct cathodic oxygen reduction, (E) on Fig. 1, right, include the functionalisation of the electrode surface with molecular species known to be efficient O2reduction catalysts, such as metal porphyrins or phthalocyanins (F),7 or the microbial reduction of oxygen at the cathode (G).8,9 Another promising approach that has not been examined so far in the cathodic compartment of MFCs, is the use of oxygenase enzymes as efficient O2reduction catalysts (H).10 In this communication we report the effect of linking an enzymatic cathode based on the multi-copper oxygenase laccase, to the microbial anode of an MFC.
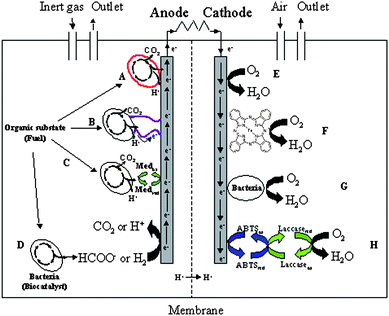 | ||
| Fig. 1 Principle of a Microbial Fuel Cell. Left: Possible mechanisms for bacterium/anode electron transfer. (A) Direct contact through outer-membrane cytochrome; (B)Electron transfer through “nanowires” or pili (a few µm long fibrous protein structures); (C) Through redox mediators (either artificial or self-produced); (D) Direct oxidation of excreted catabolites (e.g. formate, H2, etc.). Right: Paths for the reduction of oxygen into water at the cathode. (E) Direct reduction of oxygen to water, usually at a platinum electrode; (F) Through catalysis at an electrode modified by e.g. transition metal complex catalysts; (G) Through microbial catalysis; (H) Through enzymatic catalysis with an oxygenase, assisted by a redox mediator. In this study laccase from T. versicolor and ABTS (2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt. | ||
The electron accepting site of laccase enzymes is a copper ion of the so-called T1-type whose redox potential is highly dependent on the enzyme source, and may span approximately 400 mV from ca. 400 to 800 mV vs. NHE.11 One of the highest redox potential known (E0′ = 780 mV vs. NHE) is found in laccase from the fungus Trametes versicolor. Laccase (T. versicolor) has also been used for glucose/O2 enzymatic fuel cells despite its maximun activity at pH 5, low activity at physiological pH, and its relative sensitivity to chloride.12–15 In order to efficiently mediate electron transfer between the cathode and laccase (T. versicolor), the well suited ABTS redox mediator has been used (ABTS is 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt, with E0′ = 0.66 to 0.72 V vs. NHE at pH 4 to 7).12
The MFC design used in this study consisted of two glass bottles linked by an agar salt bridge (NaCl 100 mM). A mixture of soil and garden compost was extracted16 with a 10 mM NaCl solution, filtered, and 350 mL was used to fill the air-tight anodic compartment containing a rectangular glassy carbon electrode (2 × 5 cm). D-glucose and acetate were added in the anolyte to reach a concentration of 10 mM. The cathodic compartment was filled with an equal volume of 10 mM NaCl solution, it was continuously saturated with air, and contained a platinum electrode of geometry similar to that of the anode. The two electrodes were then connected through an external resistor (4.7 kΩ) and the open circuit potential (OCP) of the MFC was sampled over time with a multimeter, with the resistor disconnected.
In a typical experiment, the MFC displayed a low OCP (ca. 100 mV) for about two days before it gradually increased to ca. 600 mV after a week. This behavior is consistent with oxygen depletion in the anolyte concomitant with the development of exoelectrogenic bacterial activity at the anode.16 Once the OCP of the MFC peaked, the catholyte was then replaced by a pH 5 0.1 M acetate buffer, an electrolyte suitable for optimum activity of laccase (T. versicolor). The MFC performance could then be measured and is reported in Fig. 2 as the power density in mW m−2, with respect to the geometrical area of the electrode, against the fuel cell potential, ΔE, in Volt. Fig. 2 (circles) shows that the maximum power density of the control fuel cell was 4.6 mW m−2 at 0.20 V. The modest power density of the MFC is consistent with its high internal resistance1 (ca. 3 kΩ, a value expected for this kind of fuel cell design) and its OCP (600 mV) reflects the relatively high oxygen reduction overpotential at the cathode.
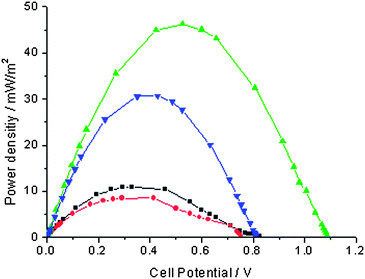 | ||
| Fig. 2 Effect of adding the laccase/ABTS catalyst in the catholyte (pH 5 acetate buffer) of a microbial fuel cell and comparison with the addition of ferrcyanide. Circles: control with the platinum cathode in pH 5 acetate buffer. Squares: after addition of ABTS (2 mM). Triangles, pointing up: after addition of ABTS (2 mM) and laccase (580 units L−1). Triangles, pointing down: ferricyanide 50 mM. | ||
Addition of 2 mM ABTS (in its fully reduced state) to the catholyte resulted in an expected increased OCP (by ca. 200 mV at 800 mV) but did not significantly increase the maximum power density, Fig. 2 (squares). Upon addition of laccase, however, there was a sharp 10-fold increase of the maximum power density that reached 46.3 mW m−2 at the higher voltage of 0.50 V, Fig. 2 (triangles, pointing up). Addition of 233 active units of laccase was actually sufficient to reach the maximum power ouput in the presence of 2 mM redox mediator. The OCP was as high as 1.1 V in the presence of laccase and ABTS. The increased performance of the MFC was then compared with a classical mediated cathodic reaction found in the MFC literature. Although the ferricyanide/ferrocyanide couple has a lower redox potential (0.42 V vs. NHE at pH 5) than permanganate/manganese dioxide (1.3 V vs. NHE at pH 5), it was chosen because, in principle, its use could be sustainable if the reoxidation of ferrocyanide by air was fast enough, as opposed to the formation of insoluble and stable MnO2.17Fig. 2 shows that the cathodic ABTS/laccase/O2 system yields higher maximum power density compared to a cathodic reaction based on K3[Fe(CN)6]. The order of magnitude of the power output is however similar for both systems (46 mW m−2 at 0.50 V vs. 31 mW m−2 at 0.43 V, respectively) and the higher performance of the laccase-based cathode can be assigned to the higher redox potential of ABTS compared with that of ferricyanide (consistently, the OCP of both MFCs was over 200 mV apart). Not only is the laccase-based system more interesting in terms of power output, it is also potentially sustainable since it effects the reduction of freely available oxygen into water. The cyclic reoxidation of ferrocyanide to ferricyanide by air is a very slow process and is therefore not practical to implement as a sustainable process.25,26 Sustainability of the laccase-based system in a continuous-flow MFC would require the immobilisation of the catalyst at the surface of the cathode. Several approaches for the immobilization of the enzyme and/or the redox mediator system have been reported in the literature and are being developed and improved.12–15,18–23 As an illustrative example, we use the physisorption of a reticulated enzyme hydrogel, as introduced and developed by Heller and co-workers.12–15 A 500 µL drop of pH 5 acetate buffer containing the enzyme (450 active units) and the crosslinker (poly(ethylene glycol) diglycidyl ether, 25 mg) was spread on one side of the platinum electrode and let to dry and react over two days. The electrode was then let to settle one day in buffer in order to allow for the hydration of the gel and for the leaching out of any unreacted or loosely bound material at the surface. The total geometric electrode surface covered with the hydrogel was approximately 10 cm2. The MFC with the laccase bound cathode showed a low power output in the absence of the redox mediator (6.5 mW m−2 at 0.23 V) indicating that direct electron transfer from the enzyme hydrogel was absent or occurred at a very low rate. An increased performance in the presence of ABTS (37 mW m−2 at 0.47 V), Fig. 3, showed that the enzyme hydrogel is permeable to the redox mediator, oxygen and water. No change occurred in the MFC power density curve after two days of continuous operation. To make this system sustainable for a continuous-flow system would require the co-immobilisation of the redox mediator as reported in the literature12–15,18,19 or the refinement of the enzyme immobilisation method so as to increase the rate of direct electron transfer and avoid the use of a redox mediator altogether.22,28,29 Increase of the time stability of the modified electrode is obviously also an important issue for practical application.20–23,27–29
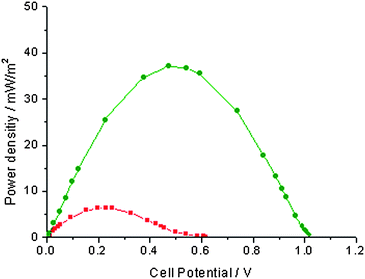 | ||
| Fig. 3 Power density curves for an MFC with a reticulated laccase physisorbed at the cathode. Squares: control with no ABTS in the catholyte (pH 5 acetate buffer). Circles: in the presence of 2 mM ABTS in the catholyte (pH 5 acetate buffer). | ||
The results reported here show that an enzymatic cathode should be considered in conjunction with a microbial anode to obtain MFCs of increased power output and sustainability. In addition to a greater maximum power density at a higher operating voltage (compared with the ferricyanide system), the laccase cathode offers the advantage of drawing on air, yields water as a product, and the enzyme/redox mediator system can be immobilized at the electrode through different approaches available in the literature.12–15,18–23 The known drawbacks of an enzymatic cathode (cost of producing the bio-catalyst, limited life-time, robustness and catalytic activity issues for the immobilised catalyst)18 need to be taken into account and addressed. In any case, the choice of a suitable cathode for MFCs should be carefully considered among the possibilities offered by chemical, enzymatic and microbial catalyses in terms of power, durability and sustainability.6–9
Experimental
Chemicals and materials
Laccase from T. versicolor (23.3 active units mg−1) was purchased from Fluka, ABTS and D-glucose from Sigma, potassium ferricyanide and sodium chloride from Acros and sodium acetate from Prolabo.The compost mixture was made with 2 L of 10 mM NaCl solution, 700 mL of (solid) commercial soil and 300 mL of (solid) garden compost. After one hour of maceration the mixture was coarsely filtered and used to fill the anodic compartment of our MFC and D-glucose and sodium acetate were added to reach a concentration of 10 mM.
Power density was calculated from the measurement of the cell voltage under different external loads using a Velleman DVM9912 multimeter and a Gentrad DR07 variable resistor.
Bioelectrochemical device
The H-type MFC was built by linking two bottle (350 mL each) by an agar salt bridge of 7 cm length and 1 cm diameter (100 mM NaCl). The anodic compartment was filled with the compost mixture described earlier and the electrode was made out glassy carbon (2 cm × 5 cm). The cathodic compartment using a platinum electrode (2 cm × 5 cm) was first filled with 200 mL of a 10 mM NaCl solution. Once the MFC reached stable potential (around 600 mV) the cathodic compartment was drained and refilled with the pH 5 0.1M acetate buffer used in this study.Acknowledgements
O. S. thanks ADEME and Région Bretagne for a studentship.References
- B. E. Logan, Microbial Fuel Cells, Wiley-VCH, Weinheim, 2008 Search PubMed.
- M. Rosenbaum, F. Zhao, U. Schröder and F. Scholz, Angew. Chem., Int. Ed., 2006, 45, 6658–6661 CrossRef CAS.
- U. Schröder, Phys. Chem. Chem. Phys., 2007, 9, 2619–2629 RSC.
- R. A. Rozendal, H. V. M. Hamelers, K. Rabaey, J. Keller and C. J. N. Buisman, Tr. Biotechnol., 2008, 26, 450–459 Search PubMed.
- P. Clauwaert, P. Aelterman, T. H. Pham, L. De Schamphelaire, M. Carballa, K. Rabaey and W. Verstaete, Appl. Microbiol. Biotechnol., 2008, 26, 450–459.
- M. L'Her, Redox properties, electrochemistry of oxygen, in Encyclopedia of Electrochemistry: Inorganic Electrochemistry, ed. A. J. Bard, M. Stratman, F. Scholz, C. J. Pickett, Wiley-VCH, Weinheim, 2006, vol. 7a, pp. 119–142 Search PubMed.
- F. Zhao, F. Harnisch, U. Schröder, F. Scholz, P. Bogdanoff and I. Herrmann, Electrochem. Commun., 2005, 7, 1405–1410 CrossRef CAS.
- K. Rabaey and J. Keller, Water Sci. Technol., 2008, 57, 655–659 Search PubMed.
- K. Rabaey, S. T. Read, P. Clauwaert, S. Freguia, P. L. Bond, L. L. Blackall and J. Keller, ISME J., 2008, 2, 519–527 CrossRef CAS.
- E. I. Solomon, A. J. Augustine and J. Yoon, Dalton Trans., 2008, 3921–3932 RSC.
- S. Sadhasivam, S. Savitha, K. Swaminathan and F.-H. Lin, Process Biochem., 2008, 43, 736–742 CrossRef CAS.
- S. Calabrese Barton, J. Gallaway and P. Atanassov, Chem. Rev., 2004, 104, 4867–4886 CrossRef CAS.
- V. Soukharev, N. Mano and A. Heller, J. Am. Chem. Soc., 2004, 126, 8368–8369 CrossRef CAS.
- F. Barrière, Y. Ferry, D. Rochefort and D. Leech, Electrochem. Commun., 2004, 6, 237–241 CrossRef.
- F. Barrière, P. Kavanagh and D. Leech, Electrochim. Acta, 2006, 51, 5187–5192 CrossRef CAS.
- S. Parot, M.-L. Délia and A. Bergel, Bioresour. Technol., 2008, 99, 4809–4816 CrossRef CAS.
- S. You, Q. Zhao, J. Zhang, J. Jiang and S. Zhao, J. Power Sources, 2006, 162, 1409–1415 CrossRef CAS.
- M. J. Cooney, V. Svoboda, C. Lau, G. Martin and S. D. Minteer, Energy Environ. Sci., 2008, 1, 320–337 RSC.
- O. Schaetzle, F. Barrière and K. Baronian, Energy Environ. Sci., 2008 10.1039/b810642h.
- M. Pellissier, F. Barrière, A. J. Downard and D. Leech, Electrochem. Commun., 2008, 10, 835–838 CrossRef CAS.
- S. Boland, F. Barrière and D. Leech, Langmuir, 2008, 24, 6351–6358 CrossRef CAS.
- C. Vaz-Dominguez, S. Campuzano, O. Rüdiger, M. Pita, M. Gorbacheva, S. Shleev, V. M. Fernandez and A. L. De Lacey, Biosens. Bioelectron., 2008, 24, 531–537 CrossRef CAS.
- G. Merle, L. Brunel, S. Tingry, M. Cretin, M. Rolland, K. Servat, C. Jolivalt, C. Innocent and P. Seta, Mater. Sci. Eng., C, 2008, 28, 932–938 CrossRef CAS.
- G. Reguera, K. P. Nevin, J. S. Nicoll, S. F. Covalla, T. L. Woodard and D. R. Lovley, Appl. Environ. Microbiol., 2006, 72, 7345–7348 CrossRef CAS.
- Z. He and L. T. Angement, Electroanalysis, 2006, 18, 2009–2015 CrossRef CAS.
- A. ter Heijne, H. V. M. Hamelers and C. J. N. Buisman, Environ. Sci. Technol., 2007, 41, 4130–4134 CrossRef CAS.
- M. Smolander, H. Boer, M. Valkiainen, R. Roozeman, M. Bergelin, J.-E. Eriksson, X.-C. Zhang, A. Koivula and L. Viikari, Enzyme Microb. Technol., 2008, 42, 93–102 CrossRef.
- C. F. Blanford, R. S. Heath and F. A. Armstrong, Chem. Commun., 2007, 1710–1712 RSC.
- J. A. Cracknell, K. A. Vincent and F. A. Armstrong, Chem. Rev., 2008, 108, 2439–2461 CrossRef CAS.
- As pointed out by one referee it is important to realize that, depending on the cathode material (e.g. those based on carbon) and potential, and on the pH conditions, hydrogen peroxide may be the major product through the two-electron reduction of oxygen. In this context enzymes that specifically catalyze the four-electron reduction of oxygen to water at high potential (e.g. laccase from T. versicolor) are very interesting.
| This journal is © The Royal Society of Chemistry 2009 |
