Shell crosslinked nanoparticles carrying silver antimicrobials as therapeutics†
Yali
Li
a,
Khadijah
Hindi
b,
Kristin M.
Watts
b,
Jane B.
Taylor
b,
Ke
Zhang
a,
Zicheng
Li
a,
David A.
Hunstad
*bc,
Carolyn L.
Cannon
*bc,
Wiley J.
Youngs
*d and
Karen L.
Wooley
*ae
aDepartments of Chemistry and Radiology, Washington University, St. Louis, MO 63130-4899, USA
bDepartment of Pediatrics, Washington University School of Medicine, St. Louis, MO 63110-1002, USA. E-mail: cannon_c@kids.wustl.edu; Fax: +1 314 286-2895; Tel: +1 314 286-2954
cDepartment of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110-1002, USA. E-mail: hunstad_d@kids.wustl.edu; Fax: +1 314 286-2895; Tel: +1 314 286-2874
dDepartment of Chemistry and Center for Silver Therapeutics Research, University of Akron, Akron, OH 44325-3601, USA. E-mail: youngs@uakron.edu; Fax: +1 330 972-7370; Tel: +1 330 972-5362
eDepartment of Chemistry and Department of Chemical Engineering, Texas A&M University, College Station, TX 77842-3012, USA. E-mail: wooley@mail.chem.tamu.edu; Fax: +1 979 862-1137; Tel: +1 979 845-4077
First published on 9th November 2009
Abstract
Amphiphilic polymer nanoparticles loaded with silver cations or/and N-heterocyclic carbene–silver complexes were assessed as antimicrobial agents against Gram-negative pathogens Escherichia coli and Pseudomonas aeruginosa.
Silver has long been prized as an antimicrobial; ancient Egyptians used silver in food storage. In the present day, silver compounds are widely used as antimicrobial agents, especially in the treatment of wounds and burns.1,2 Silver cation (Ag+) is highly toxic, or described as “oligodynamic,” against a broad spectrum of microorganisms, probably because of its inhibition of certain oxidative enzymes, protein denaturation, or interference with DNA replication.3 Unlike traditional antibiotics, Ag+ is of low toxicity to human tissues and has elicited only rare instances of bacterial silver resistance.4–6 A variety of silver-based antimicrobials, therefore, has been synthesized and evaluated. Fox introduced silver sulfadiazine (SSD) in the 1960s;7 SSD remains routinely used as a topical treatment of burns. Although it has been recognized as an antimicrobial since at least the 1800s, Moyer repopularized AgNO3 for treatment of burns, which prompted development of SSD.2 Unfortunately, AgNO3 is not practical in vivo, because Ag+ complexes with salts and other biological agents in the bloodstream.7 Over the past decade, an array of silver N-heterocyclic carbene (NHC) complexes, which exhibit improved stability to light and aqueous solution, have been synthesized and investigated by Youngs and Cannon as potential antimicrobial agents and have shown very promising results with both in vitro and in vivo studies in a variety of bacteria including BSL3 organisms.8–11 Small molecule antibiotics also have a major problem, however, that of rapid clearance from the human body and, in the case of silver, reaction with sulfur-containing proteins and chloride in the bloodstream.12 Therefore, there remains a need for packaging and protection for therapeutic delivery of Ag+ or silver–carbene complexes (SCCs).
Silver-containing (mostly silver(0) nanoparticles) polymers,13 hyperbranched polymers,14 and dendrimers15 have been investigated for improved solubility and processability to form antimicrobial surfaces for biomaterial-related infections. Youngs and Cannon et al. had previously loaded SCCs into L-tyrosine polyphosphate nanoparticles and demonstrated potent antimicrobial efficacy in in vitro and in vivo studies against Pseudomonas aeruginosa.16 In this study, we developed shell crosslinked knedel-like (SCK) nanoparticles17–20 as an antimicrobial device, designed to encapsulate and protect Ag+, SCCs, or the two agents coincidentally, and evaluate the relative efficacy of each system. The SCKs were constructed by the supramolecular assembly of amphiphilic block copolymers, poly(acrylic acid)-b-polystyrene (PAA-b-PS), into micelles, followed by covalent crosslinking throughout the shell layer to afford discrete nanostructures having a hydrophobic core domain and a hydrophilic shell region. Four procedures were then followed for loading of the SCKs with silver: (1) Ag+ was incorporated from AgNO3 into the hydrophilic PAA shell region (AgNO3–SCK); (2) 1-hexyl-3-methyl-4,5-dichloro-imidazole-2-ylidene silver(I) acetate (SCC10, which undergoes decomposition in the presence of saline solution to release active Ag+)21 was loaded into the hydrophobic PS core domain and/or the core–shell interface (SCC10–SCK);22,23 (3) and (4) both methods were applied in opposite order of addition (AgNO3–SCC10–SCK or SCC10–AgNO3–SCK) (Fig. 1). In all cases, free silver was eliminated using a centrifugal filter device (100 kDa MWCO), and the filtrates were examined by UV-visible spectroscopy to confirm removal of free silver. The resulting silver-bearing nanoparticles were characterized and their antimicrobial activities against common Gram-negative pathogenic bacteria were evaluated in vitro.
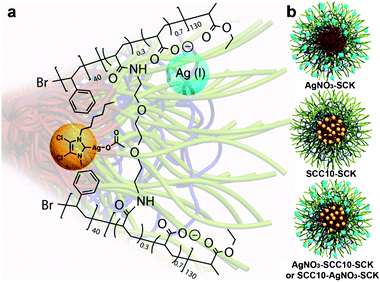 | ||
| Fig. 1 Schematic representations of (a) SCC10 (yellow ball) incorporated into the core and Ag+ (blue ball) from AgNO3 chelated into the shell of an SCK prepared from PAA130-b-PS40; and (b) AgNO3–SCK, SCC10–SCK, and AgNO3–SCC10–SCK or SCC10–AgNO3–SCK. Note: the placements of the silver species within the SCK framework are hypothetical locations, which have not been confirmed experimentally. | ||
The silver-loading capacities of the nanoparticles were measured by inductively coupled plasma-mass spectrometry (ICP-MS) using Tl as an internal standard (see ESI†). The amount of Ag loaded increased with increasing feed amounts. AgNO3–SCK reached a [Ag] loading capacity of ca. 370 μg mL−1 at the highest AgNO3 feed of 200 mol% with respect to the combined total theoretical moles of acrylic acid and amide residues in the SCK shell, whereas SCC10–SCK had a capacity of ca. 75 μg mL−1 at 200 wt% feed of SCC10 with respect to the polymer weight of the SCK solution. The efficiencies for loading, measured as the percentage of silver loaded into the SCKs vs. the amount of silver in the feed, were constant across the feed ratios, and were consistently higher for the AgNO3 loading method. Sequential silver loading by both methods (performed in either order) did not improve silver capacity over Ag+-loading only, reaching a total [Ag] of ca. 220 μg mL−1 at 150% feed. Higher feeds of silver caused precipitation. The silver-bearing nanoparticles were examined by transmission electron microscopy (TEM), and were observed to be uniform nanostructures of sizes that agreed with the non-Ag-loaded SCKs (Fig. 2). Some elemental silver nanoparticles were observed in the AgNO3–SCK sample (see ESI†), which might be due to the reduction of Ag+ to Ag(0) in the amine-containing polymer matrix.9,15,24
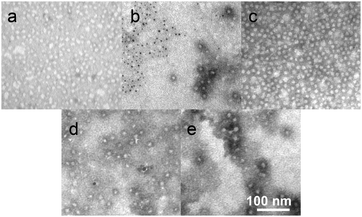 | ||
| Fig. 2 TEM images of SCKs and silver-loaded SCKs, each with negative staining by 1% phosphotungstic acid, (a) SCK, (b) AgNO3–SCK, (c) SCC10–SCK, (d) AgNO3–SCC10–SCK, and (e) SCC10–AgNO3–SCK. The scales are consistent. | ||
Release of silver from the SCK nanoparticles was assessed by monitoring the decrease over time of the concentration of silver in dialysis cassettes, performed at 37 °C in 5 mM PBS at pH 7.4 and analyzed by ICP-MS (Fig. 3). Each loading protocol gave ca. 50% release of silver within ca. 1 day and ca. 80% release within 2 days, obtaining a plateau with full silver release by ca. 4 days, a time period that would provide a desired depot effect for therapeutic delivery. Moreover, the stability of these Ag–SCK complexes over many hours in PBS is a distinct advantage, relative to simple silver salt solutions, for future in vivo studies.
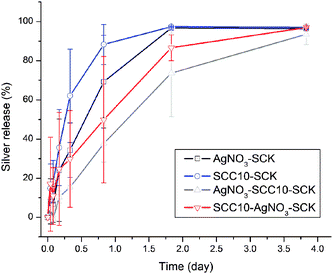 | ||
| Fig. 3 Release profiles of silver from silver-bearing nanoparticles at 37 °C in 5 mM PBS at pH 7.4 (duplicate). | ||
The antimicrobial activities of the silver-loaded nanoconstructs against common Gram-negative pathogenic bacteria were measured. We first tested the antimicrobial activity of SCC10 (in aqueous solution with 1% dimethyl sulfoxide) by determining the minimal inhibitory concentration (MIC) in Mueller–Hinton (MH) broth against urinary isolates of Escherichia coli and respiratory isolates of P. aeruginosa from patients with cystic fibrosis. These MICs were physiologically relevant, ranging from 1 to 6 μg mL−1 (see ESI†). As positive and negative controls, the MICs of SCC10 against E. coli strain J53 with and without the silver resistance plasmid pMG1015,6 were tested. The MIC of SCC10 was 1 μg mL−1 for J53 but >10 μg mL−1 for J53/pMG101, demonstrating that the antimicrobial activity of SCC10 is conferred by the silver moiety.
Next, we tested the activity of our silver-bearing SCK constructs against representative strains of E. coli (strain UTI89; MIC [SCC10] = 2 μg mL−1) and P. aeruginosa (strain PAM57-15; MIC [SCC10] = 1 μg mL−1). Defined suspensions of these strains in MH broth were treated in 96-well plates with the silver-bearing SCKs, equalized for [Ag] by the ICP-MS data. Bacterial growth was measured by optical density (650 nm) in a microplate spectrophotometer 6 h after treatment. SCKs without loaded silver had no antimicrobial activity (data not shown). Independent of the silver-loading method, decrements in growth of E. coli UTI89 were observed at [Ag] of 1 μg mL−1, and growth was completely inhibited at [Ag] of 2 μg mL−1 (Fig. 4a). For P. aeruginosa PAM57-15, decrements in growth were observed at [Ag] of 2–4 μg mL−1 and growth was completely inhibited at [Ag] of 8 μg mL−1 (Fig. 4b). Activity of the silver-bearing SCKs was generally inferior to that of naked AgNO3 by ≤1 two-fold dilution in inhibition of bacterial growth, suggesting that the SCKs provide availability of silver for antimicrobial action.
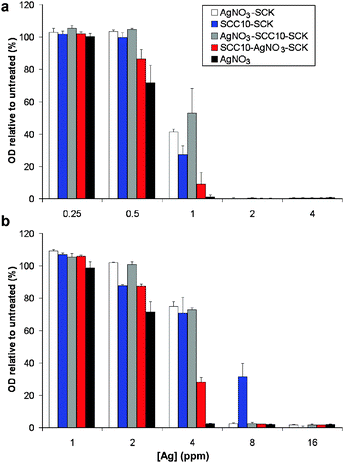 | ||
| Fig. 4 Inhibition of growth of E. coli strain UTI89 (a) and P. aeruginosa strain PAM57-15 (b) by silver-bearing nanoparticles and naked AgNO3. Relative optical density (650 nm) after 6 h is shown for each construct at the indicated silver concentrations. | ||
These silver-loaded SCK nanoparticle delivery systems exhibited antimicrobial activities, which were nearly comparable to AgNO3. There appeared to be no advantage to the use of the silver–carbene compounds vs. loading with silver cations directly. The sustained release over a period of hours suggests that these nanoparticle delivery systems may be beneficial in the treatment of microbial infections in vivo. Packaging in the nanoparticle framework is expected to provide for in vivo stability. Furthermore, they can be functionalized, which may permit control over biodistribution,25 tissue-selective targeting26 and in vivo clearance.27,28 We are currently investigating their potential in the treatment of pulmonary and urinary tract infections.
Grants in support of this work from the NIH (HL080729, GM086895, AI067856 and DK067894), the March of Dimes (Basil O'Connor grant 5-09-105), and the Johnson & Johnson Washington University Translational Research Fund are gratefully acknowledged.
Notes and references
- S. Silver, L. T. Phung and G. Silver, J. Ind. Microbiol. Biotechnol., 2006, 33, 627–634 CrossRef CAS.
- C. A. Moyer, J. Natl. Med. Assoc., 1965, 57, 95–100 Search PubMed.
- S. L. Percival, P. G. Bowler and D. Russell, J. Hosp. Infect., 2005, 60, 1–7 CrossRef CAS.
- K. Bridges, A. Kidson, E. J. L. Lowbury and M. D. Wilkins, Br. Med. J., 1979, 1, 446–449 CrossRef CAS.
- A. Gupta, K. Matsui, J.-F. Lo and S. Silver, Nat. Med., 1999, 5, 183–188 CrossRef CAS.
- S. Silver, FEMS Microbiol. Rev., 2003, 27, 341–353 CrossRef CAS.
- C. L. Fox, Arch. Surg., 1968, 96, 184–188 Search PubMed.
- J. C. Garrison and W. J. Youngs, Chem. Rev., 2005, 105, 3978–4008 CrossRef CAS.
- A. Melaiye, Z. Sun, K. Hindi, A. Milsted, D. Ely, D. H. Reneker, C. A. Tessier and W. J. Youngs, J. Am. Chem. Soc., 2005, 127, 2285–2291 CrossRef CAS.
- A. M. Kascatan-Nebioglu, A. Pas, K. Hindi, S. Durmus, M. J. Panzner, L. A. Hogue, R. J. Mallett, C. E. Hovis, M. Coughenour, S. D. Crosby, A. Milsted, D. L. Ely, C. A. Tessier, C. L. Cannon and W. J. Youngs, J. Med. Chem., 2006, 49, 6811–6818 CrossRef CAS.
- (a) K. M. Hindi, T. J. Siciliano, S. Durmus, M. J. Panzner, D. A. Medvetz, D. V. Reddy, L. A. Hogue, C. E. Hovis, J. K. Hilliard, R. J. Mallet, C. A. Tessier, C. L. Cannon and W. J. Youngs, J. Med. Chem., 2008, 51, 1577–1583 CrossRef CAS; (b) K. M. Hindi, M. J. Panzner, C. L. Cannon and W. J. Youngs, Chem. Rev., 2009, 109(8), 3859–3884 CrossRef CAS; (c) M. J. Panzner, A. Deeraksa, A. Smith, B. D. Wright, K. M. Hindi, A. Kascatan-Nebioglu, A. G. Torres, B. M. Judy, C. E. Hovis, J. K. Hilliard, R. J. Mallett, E. Cope, D. M. Estes, C. L. Cannon, J. G. Leid and W. J. Youngs, Eur. J. Inorg. Chem., 2009, 1739–1745 CrossRef CAS; (d) M. J. Panzner, K. M. Hindi, B. D. Wright, J. B. Taylor, D. S. Han, W. J. Youngs and C. L. Cannon, Dalton Trans., 2009, 7308–7313 RSC; (e) C. L. Cannon, L. A. Hogue, R. K. Vajravelu, G. H. Capps, A. Ibricevic, K. M. Hindi, A. Kascatan-Nebioglu, M. J. Walter, S. L. Brody and W. J. Youngs, Antimicrob. Agents Chemother., 2009, 53(8), 3285–3293 CrossRef.
- P. Meers, M. Neville, V. Malinin, A. W. Scotto, G. Sardaryan, R. Kurumunda, C. Mackinson, G. James, S. Fisher and W. R. Perkins, J. Antimicrob. Chemother., 2008, 61, 859–868 CrossRef CAS.
- V. Sambhy, M. M. MacBride, B. R. Peterson and A. Sen, J. Am. Chem. Soc., 2006, 128, 9798–9808 CrossRef CAS.
- C. Aymonier, U. Schlotterbeck, L. Antonietti, P. Zacharias, R. Thomann, J. C. Tiller and S. Mecking, Chem. Commun., 2002, 3018–3019 RSC.
- L. Balogh, D. R. Swanson, D. A. Tomalia, G. L. Hagnauer and A. T. McManus, Nano Lett., 2001, 1, 18–21 CrossRef CAS.
- K. M. Hindi, A. J. Ditto, M. J. Panzner, D. A. Medvetz, D. S. Han, C. E. Hovis, J. K. Hilliard, J. B. Taylor, Y. H. Yun, C. L. Cannon and W. J. Youngs, Biomaterials, 2009, 30, 3771–3779 CrossRef CAS.
- D. Pan, J. L. Turner and K. L. Wooley, Chem. Commun., 2003, 2400–2401 RSC.
- K. Qi, Q. G. Ma, E. E. Remsen, C. G. Clark and K. L. Wooley, J. Am. Chem. Soc., 2004, 126, 6599–6607 CrossRef CAS.
- X. K. Sun, R. Rossin, J. L. Turner, M. L. Becker, M. J. Joralemon, M. J. Welch and K. L. Wooley, Biomacromolecules, 2005, 6, 2541–2554 CrossRef CAS.
- A. M. Nyström, Z. Q. Xu, J. Q. Xu, S. Taylor, T. Nittis, S. A. Stewart, J. Leonard and K. L. Wooley, Chem. Commun., 2008, 3579–3581 RSC.
- D. A. Medvetz, K. M. Hindi, M. J. Panzner, A. J. Ditto, Y. H. Yun and W. J. Youngs, Met. Based Drugs, 2008, 2008, 384010 Search PubMed.
- H.-M. Kao, R. D. O’Connor, A. Mehta, H. Huang, B. Poliks, K. L. Wooley and J. Schaefer, Macromolecules, 2001, 34, 544–546 CrossRef CAS.
- H. Huang, K. L. Wooley and J. Schaefer, Macromolecules, 2001, 34, 547–551 CrossRef CAS.
- S. H. Im, Y. T. Lee, B. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2154–2157 CrossRef.
- G. Sun, A. Hagooly, J. Xu, A. M. Nyström, Z. Li, R. Rossin, D. A. Moore, K. L. Wooley and M. J. Welch, Biomacromolecules, 2008, 9, 1997–2006 CrossRef CAS.
- A. Almutairi, R. Rossin, M. Shokeen, A. Hagooly, A. Ananth, B. Capoccia, S. Guillaudeu, D. Abendschein, C. J. Anderson, M. J. Welch and J. M. J. Fréchet, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 685–690 CrossRef CAS.
- E. D. Pressly, R. Rossin, A. Hagooly, K. Fukukawa, B. W. Messmore, M. J. Welch, K. L. Wooley, M. S. Lamm, R. A. Hule, D. J. Pochan and C. J. Hawker, Biomacromolecules, 2007, 8, 3126–3134 CrossRef CAS.
- K. Fukukawa, R. Rossin, A. Hagooly, E. D. Pressly, J. N. Hunt, B. W. Messmore, K. L. Wooley, M. J. Welch and C. J. Hawker, Biomacromolecules, 2008, 9, 1329–1339 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Complete methods, particle characterization data, and a table of MIC values for tested bacterial strains. See DOI: 10.1039/b916559b |
| This journal is © The Royal Society of Chemistry 2010 |
