Hydrophobic chromophore cargo in micellar structures: a different strategy to sensitize lanthanide cations†
Célia S.
Bonnet
a,
Laurent
Pellegatti
b,
Frédéric
Buron
b,
Chad M.
Shade
c,
Sandrine
Villette
a,
Vojtěch
Kubíček
a,
Gérald
Guillaumet
b,
Franck
Suzenet
b,
Stéphane
Petoud
*ac and
Éva
Tóth
*a
aCentre de Biophysique Moléculaire, CNRS, rue Charles Sadron, 45071 Orléans, France. E-mail: eva.jakabtoth@cnrs-orleans.fr
bInstitut de Chimie Organique et Analytique, Université d’Orléans, UMR CNRS 6005, rue de Chartres, 45067 Orléans, France
cDepartment of Chemistry, The University of Pittsburgh, 219 Parkman Avenue, 15260 Pittsburgh, USA
First published on 20th November 2009
Abstract
We propose a new approach for the versatile sensitization of luminescent lanthanide cations. A hydrophobic chromophore is incorporated into a micellar assembly formed by the amphiphilic lanthanide chelate. The sensitizer to lanthanide energy transfer occurs between the two moieties without covalent linkage.
Lipid-based colloidal aggregates such as liposomes, micelles or microemulsions are gaining importance in molecular imaging since they allow for a marked sensitivity enhancement due to the great payload of magnetic or optical reporters within a single particle.1,2 Their further functionalization can provide biocompatibility or targeting ability.1,2 Amphiphilic systems are also extensively exploited in vivo as carriers of hydrophobic drugs.3 Micellar aggregations allow exploiting dynamic interactions promoted between molecules being part of the same micelle, even if they are not linked by a covalent bond. The micelle and its molecular content behave as a self-assembled, multicomponent nanosized device. For instance, intramicellar energy and electron transfer can be realized between a fluorophore and a lipophilic metal complex to create molecular logic gates,4 fluorescent micellar sensors for ions5 or pH.6
Lanthanide complexes are used as luminescent reporters in several biological applications.7 They require a chromophore to sensitize their luminescence in order for the compound to emit a sufficient number of photons.8 To ensure efficient energy transfer, this chromophore needs to be placed in close proximity of the luminescent cation. Classically, this goal is achieved by covalently linking the appropriate chromophore to the metal chelating moiety which often requires difficult and time-consuming synthetic procedures. The efficiency of the chromophore can only be determined by the test of the final compound.
We propose here an alternative strategy for the rapid development and screening of luminescent lanthanide compounds and sensitizers. Our approach is to incorporate the hydrophobic chromophore in a micelle that consists of amphiphilic chelates of the luminescent lanthanide and to use the energy transfer between the two non-covalently linked moieties (Fig. 1). This concept combines several advantages: (i) the use of hydrophobic chromophores including large aromatic moieties with a high level of conjugation that are poorly soluble in aqueous solution, (ii) the use of chromophores that do not possess binding groups for the formation of coordination bonds with the lanthanide cations, (iii) the formation of polymetallic lanthanide compounds. These micelles contain both the lanthanide and the chromophore in a large amount which allows maximizing the overall absorbance and the number of emitted photons per unit of volume for more sensitive detection even with low quantum yields.
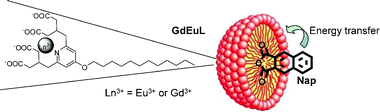 | ||
| Fig. 1 Schematic representation of the micelle with the inclusion of the naphthalimide. | ||
Hydrophobic fluorescence dyes, like pyrene derivatives, exhibit different fluorescence characteristics depending upon the properties of the solubilizing medium and have been used for a long time to study micellization.9 More recently, aggregation induced emission of fluorophores has been exploited to monitor micelle formation.10 On the other hand, in DELFIA dissociative luminescent assays, amphiphilic surfactants are commonly used to protect the lanthanide compound from dissociation and from non-radiative deactivation in a micelle-like structure.11 We emphasize that our strategy is fundamentally different from any of these approaches since it takes advantage of the micellar structure as a platform to integrate both the lanthanide complex and the chromophore in close proximity, without covalent linkage.
We have recently described bimodal luminescent and MRI contrast agents formed with pyridine-derivative ligands.12 This chelator simultaneously provides high MRI efficiency for the Gd3+ complex (two inner sphere water molecules) and remarkable optical properties for near-infrared emitting lanthanides. The amphiphilic chelate obtained by conjugation of a C12 hydrophobic chain to the pyridine core (synthesis in ESI†) forms micelles in aqueous solution. A bimetallic micellar system with 10% Eu3+ and 90% Gd3+, GdEuL, has been prepared. A hydrophobic chromophore (10% with respect to the ligand) was incorporated within the micelle by sonication of the aqueous micellar solution (9.43 mM overall Ln3+ concentration). 2,3-Naphthalimide (Nap) was chosen as the chromophore as it cannot coordinate to the lanthanide, and can only interact through hydrophobic interactions with the micelle. It is also known as a sensitizer for Eu3+ (Fig. 1).13 The micellar solution readily dissolves the Nap which is otherwise poorly water soluble.
Micelle formation is characterized by the critical micellar concentration (cmc), above which micelles exist in solution. The presence of Gd3+ allowed for determining the cmc of GdEuL–Nap by 1H relaxivity measurements (40 MHz and 25 °C; ESI†). It is 1.15 ± 0.05 mM which, in comparison to previously reported analogous amphiphilic Gd3+ complexes, falls in the lower range expected for a C12 tail, due to the presence of the rigid pyridine ring.14 It is also lower than that for GdL without Nap (1.48 ± 0.05 mM) since the presence of the hydrophobic Nap promotes micellar aggregation. The number of inner sphere water molecules has been obtained by luminescence lifetime measurements on EuL. As the parent complex,12EuL is bishydrated, independently of the concentration above or under the cmc (ESI†).
The ability of Nap to sensitize Eu3+ has been investigated in the aqueous micellar solution at pH 7.0. Time-delayed emission spectra were recorded in the presence and in the absence of Nap under and above the cmc, with excitation at 345 nm (Fig. 2). The characteristic Eu3+ emission bands are present even in the absence of Nap since the pyridine moiety of the chelate also has a sensitizing effect. Nevertheless, it is clearly demonstrated that, above the cmc, the Eu3+ emission is enhanced by ca. 400% in the presence of Nap, while no enhancement is observed under the cmc. This unambiguously proves that energy transfer between the Nap and Eu3+ does occur if they are sufficiently close to each other, with the Nap trapped in the micellar structure. It is further confirmed by recording the time-delayed excitation spectra by monitoring the Eu3+ emission at 614 nm (Fig. 3). The excitation bands indicative of Nap electronic structure are observed exclusively above the cmc (see ESI for UV spectra†).
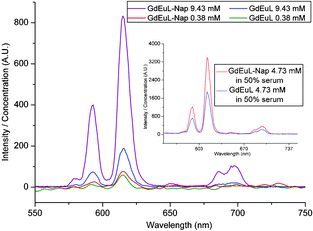 | ||
| Fig. 2 Time-delayed emission spectra of GdEuL recorded upon excitation at 345 nm, pH 7; time delay 0.1 ms. The inserted figure shows the emission spectra in fetal bovine serum. The concentrations mentioned are overall concentrations of the lanthanide. | ||
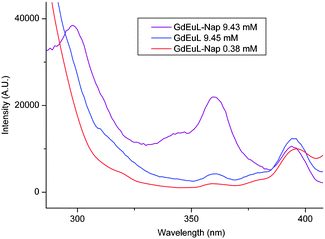 | ||
| Fig. 3 Normalized time-delayed excitation spectra of GdEuL recorded at pH 7 centered on the 614 nm Eu3+ emission. Intensities are normalized with respect to the 396 nm band (7F0 → 5L6). The concentrations mentioned are overall concentrations of the lanthanide. | ||
The efficiency of the sensitization provided by Nap to Eu3+ in the micelles was quantified by measuring the quantum yield for the system which was found to be 0.14 ± 0.02% in H2O. This quantum yield is in the same range to that reported for dendrimer-Nap systems,13 although it is important to remember that quantum yields do not reflect the absorption efficiency of the antenna.
The presence of an energy transfer from Nap to Eu3+ is a convenient means to assess if the system is in the micellar form. The time-delayed excitation and emission spectra of GdEuL–Nap have been recorded after 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dilution of the 9.43 mM solution with fetal bovine serum (FBS) and compared to the Nap-free solution (insert in Fig. 3). In FBS, like in water, there is a remarkable enhancement of the Eu3+ intensity in the presence of Nap, proving that the system stays mainly in micellar form in this biological medium. The system is stable for several hours, no significant changes are observed in the emission spectrum of the micelle in serum after one day. β-Cyclodextrin (β-CD) is known to destroy micellar aggregates.15 Luminescence spectra of GdEuL–Nap have been recorded upon addition of up to 4 equivalents of β-CD and showed a decrease in the Eu3+ emission due to destruction of the micelles (further addition of β-CD results in precipitation; ESI†).
1 dilution of the 9.43 mM solution with fetal bovine serum (FBS) and compared to the Nap-free solution (insert in Fig. 3). In FBS, like in water, there is a remarkable enhancement of the Eu3+ intensity in the presence of Nap, proving that the system stays mainly in micellar form in this biological medium. The system is stable for several hours, no significant changes are observed in the emission spectrum of the micelle in serum after one day. β-Cyclodextrin (β-CD) is known to destroy micellar aggregates.15 Luminescence spectra of GdEuL–Nap have been recorded upon addition of up to 4 equivalents of β-CD and showed a decrease in the Eu3+ emission due to destruction of the micelles (further addition of β-CD results in precipitation; ESI†).
In summary, we have demonstrated that hydrophobic chromophores incorporated into micelles formed by amphiphilic lanthanide complexes can efficiently sensitize lanthanide luminescence. This simple and rapid approach allows for creating luminescent polymetallic particles with a high number of integrated chromophores and lanthanide cations to maximize the luminescence intensity. It also offers a convenient way of screening a variety of hydrophobic chromophores including those with large, non-soluble aromatic moieties, without important synthetic effort. Finally, this strategy can be applied to assess the fate of micellar systems in various types of biological media.
This work was financially supported by the Institut National du Cancer, La Ligue contre le Cancer, France, and was carried out within the COST Action D38.
Notes and references
- M. J. W. Mulder, G. J. Strijkers, G. A. F. van Tilborg, A. W. Griffioen and K. Nicolay, NMR Biomed., 2006, 19, 142 CrossRef CAS.
- D. Delli Castelli, E. Gianolio, S. Geninatti Crich, E. Terreno and S. Aime, Coord. Chem. Rev., 2008, 252, 2424 CrossRef.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145 CrossRef CAS.
- S. Uchiyama, G. D. McClean, K. Iwai and A. P. de Silva, J. Am. Chem. Soc., 2005, 127, 8920 CrossRef CAS.
- P. Grandini, F. Mancin, P. Tecilla, P. Scrimin and U. Tonellato, Angew. Chem., 1999, 111, 3247 CrossRef.
- Y. A. Diaz-Fernandez, F. Foti, C. Mangano, P. Pallavicini, S. Patroni, A. Perez-Gramatges and S. Rodriguez-Calvo, Chem.–Eur. J., 2006, 12, 921 CrossRef CAS.
- J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC; D. Parker, Chem. Rev., 2002, 102, 1977 CrossRef CAS; C. M. G. Dos Santos, A. J. Harte, S. J. Quinn and T. Gunnlaugsson, Coord. Chem. Rev., 2008, 252, 2512 CrossRef CAS.
- S. I. Weissman, J. Chem. Phys., 1942, 10, 214–217 CrossRef CAS.
- K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039 CrossRef CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC.
- I. Hemmila, in High Throughput Screening, ed. J. Delvin, Marcel Dekker, New York, 1997, p. 361 Search PubMed.
- L. Pellegatti, J. Zhang, B. Drahos, S. Villette, F. Suzenet, G. Guillaumet, S. Petoud and E. Tóth, Chem. Commun., 2008, 6591 RSC.
- J. P. Cross, M. Lauz, P. D. Badger and S. Petoud, J. Am. Chem. Soc., 2004, 126, 16278 CrossRef CAS.
- G. M. Nicolle, E. Toth, K. P. Eisenwiener, H. R. Mäcke and A. E. Merbach, JBIC, J. Biol. Inorg. Chem., 2002, 7, 757 CrossRef CAS.
- E. Junquera, L. Peña and E. Aicart, Langmuir, 1997, 13, 219 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, photophysical and relaxometric measurements. See DOI: 10.1039/b918881a |
| This journal is © The Royal Society of Chemistry 2010 |
