Dendrimer adjusted nanocrystals of DAST: organic crystal with enhanced nonlinear optical properties†
Mei-Ling
Zheng
ab,
Wei-Qiang
Chen
a,
Katsumasa
Fujita
*b,
Xuan-Ming
Duan
*a and
Satoshi
Kawata
b
aLaboratory of Organic NanoPhotonics and Key Laboratory of Photochemical Conversion & Functional Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: xmduan@mail.ipc.ac.cn; Fax: + 86 10 8254 3597
bDepartment of Applied Physics, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: fujita@ap.eng.osaka-u.ac.jp
First published on 4th May 2010
Abstract
Organic crystals of trans-4-[4-(dimethylamino)-N-methylstilbazolium] p-tosylate (DAST) with enhanced nonlinear optical properties were successfully prepared at the nanometre scale due to the influence of a carbosiloxane dendrimer (CSiO-D).
Organic nanocrystals (NCs), the localized intermediate state between isolated molecules and bulk crystals, are attracting rapidly growing interest as advanced materials because of their unique physical properties that can be modulated not only by the functional groups within the molecules but also by the particle size and shape.1 It has been proposed that organic NCs could exhibit enhanced nonlinear optical (NLO) properties over the bulk crystals of those organic nonlinear molecules.2 As a typical organic salt, the NLO properties of trans-4-[4-(dimethylamino)-N-methylstilbazolium] p-tosylate (DAST) and its derivatives have been closely investigated from the perspective of both individual molecules3 and its bulk crystals.4 These organic salts exhibit larger NLO response and better photo/thermal-stability compared to their neutral analogues.2–4 DAST NCs, which combine the unusual properties of NCs with a strong nonlinear optical response, have been studied for their large response to the electric or magnetic fields.2,5 However, only submicrometre DAST NCs have been achieved. It is difficult to control the size and shape of DAST NCs during the preparation because of the propensity of DAST to crystallize.6 Consequently, the NCs of DAST and related derivatives generally form through rapid crystal growth processes. Therefore, adjusting the growth process of the NCs is critically important to controlling their size and shape, allowing for the discovery of their unique properties.
One of the promising strategies for adjusting the kinetic growth process of organic NCs is to use the size-isolation effect of dendrimers.7 Dendrimers possess a highly organized interior with a semi-rigid molecular construction that results in prearranged geometry and dimensions. Poly(amidoamine) (PAMAM) dendrimers have commonly been used to adjust the kinetic growth process of organic NCs of neutral molecules. For example, the 3.5 generation (G3.5) of PAMAM dendrimer, with a hydrophilic interior and hydrophobic periphery, was successfully used to control the size and shape of microcrystals of 4-n-octylamino-7-nitrobenz-2-oxa-1,3-diazole (NBD-C8).8
Silicon-containing dendrimers exhibit unique hybrid structures between inorganic and organic materials, which result in excellent chemical and thermal stability.7 Carbosiloxane dendrimers (CSiO-D) have an inorganic–organic hybrid dendritic structure including thermostable Si–C and flexible Si–O backbones as well as a hydrophobic interior. We confirmed that the hydrophobic interior and organic–inorganic hybrid properties of CSiO-D improved the photostability of laser dye molecules during the irradiation with laser pulses.9a It has also been found that CSiO-D aggregate at a low concentration of 10−5 M.9b These properties of CSiO-D imply that they could play important roles not only in adjusting the growth process of organic NCs to realize size and shape control, but also in achieving organic NCs with high photostability. This is important for the applications of NCs in the fields of nonlinear optics as well as organic laser emission.
In this paper, DAST is used to prepare organic NCs because of its excellent NLO properties. The effect of CSiO-D on the crystal growth process of DAST NCs are investigated using UV-vis spectroscopy as well as scanning electron microscopy (SEM). Nanometre-sized DAST NCs are successfully achieved due to the effective adjustment of CSiO-D in the crystal growth process. The DAST NCs prepared possess excellent NLO properties, with potential applications in the fields of nonlinear optics and fluorescent probes for nanoimaging. Significantly, the results obtained represent a facile approach for controlling the size and shape of organic NCs. This approach could be widely applied to control the crystal growth processes used for preparing organic NCs, particularly for compounds that crystallize readily.
The molecular structures of DAST and the fifth generation carbosiloxane dendrimer CSiO-G5 are shown in Scheme 1; these compounds were synthesized according to the reported procedures.10,11 DAST NCs were prepared using the well-known reprecipitation method.12 CSiO-G5 was used to improve the reprecipitation method and to form a stable dispersion of DAST NCs. A typical procedure for the preparation of DAST NCs is as follows: 50–100 μL of DAST methanol solution was injected rapidly into 10 mL of diethyl ether. The mixture was stirred vigorously for 30 s to allow complete solvent mixing, and then the mixture was ripened at room temperature.
 | ||
| Scheme 1 Chemical structures of DAST and CSiO-G5. | ||
The size and shape of the DAST NCs formed are mainly affected by the concentration and volume of DAST methanol solution added. Injecting 100 μL of 5 × 10−3 M DAST methanol solution into 10 mL of diethyl ether, the average size of DAST NCs achieved is 183 nm with a wide size distribution of 60–320 nm as shown in Fig. 1a and 1c. In this case, DAST NCs with various sizes and shapes are obtained (Fig. 1a), which is the same result as previously reported.2 The effect of CSiO-G5 on the size and shape of DAST NCs is clearly exhibited when CSiO-G5 is included in the reprecipitation process. Fig. 1b shows an SEM image of the DAST NCs prepared by injecting 100 μL of 5 × 10−3 M DAST methanol solution into a 5 × 10−5 M solution of CSiO-G5 in diethyl ether, which gives a molar ratio of CSiO-G5/DAST of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Although the average size of DAST NCs is not significantly reduced, the size distribution of the DAST NCs obtained narrows significantly compared with those prepared without CSiO-G5 (Fig. 1d). In this case, all of the DAST NCs obtained possess a regular cubic shape, which is the same as bulk DAST crystals.4 This result implies that the presence of CSiO-G5 helps slow the growth process of DAST NCs, resulting in regular DAST NCs.
1. Although the average size of DAST NCs is not significantly reduced, the size distribution of the DAST NCs obtained narrows significantly compared with those prepared without CSiO-G5 (Fig. 1d). In this case, all of the DAST NCs obtained possess a regular cubic shape, which is the same as bulk DAST crystals.4 This result implies that the presence of CSiO-G5 helps slow the growth process of DAST NCs, resulting in regular DAST NCs.
![SEM images of DAST NCs prepared a) without and b) with CSiO-G5 ([DAST] = 5 × 10−5 M, CSiO-G5/DAST = 1 : 1). c) and d) show the size distributions of the NCs in a) and b), respectively. The corresponding size of a) is 183 ± 51 nm and b) is 197 ± 24 nm.](/image/article/2010/NR/b9nr00402e/b9nr00402e-f1.gif) | ||
Fig. 1 SEM images of DAST NCs prepared a) without and b) with CSiO-G5 ([DAST] = 5 × 10−5 M, CSiO-G5/DAST = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). c) and d) show the size distributions of the NCs in a) and b), respectively. The corresponding size of a) is 183 ± 51 nm and b) is 197 ± 24 nm. 1). c) and d) show the size distributions of the NCs in a) and b), respectively. The corresponding size of a) is 183 ± 51 nm and b) is 197 ± 24 nm. | ||
Decreasing the concentration of DAST solution and the amount injected results in smaller DAST NCs in the presence of CSiO-G5. By injecting 50 μL of 4 × 10−3 M DAST methanol solution into 10 mL of diethyl ether, the average size of DAST NCs obtained is around 212 nm with a wide size distribution from 110 to 340 nm as shown in Fig. 2a and 2d. The addition of CSiO-G5 significantly reduces the size of the DAST NCs. The average sizes of the DAST NCs decrease from 212 nm to 58 and 54 nm when the molar ratios of CSiO-G5/DAST increase from 0 to 4.1 and 8.8, respectively (Fig. 2b and 2c). The size distributions of DAST NCs prepared with CSiO-G5 are also narrower than that of the NCs prepared without the dendrimer. This provides clear evidence that CSiO-G5 play a critical role in adjusting the crystal growth process of DAST NCs, allowing the realization of nanosized DAST NCs.
![SEM images of DAST NCs prepared without and with CSiO-G5. a), b) and c) CSiO-G5/DAST = 0, 4.1 and 8.8 with sizes of 212 ± 49 nm, 58 ± 7 nm and 54 ± 8 nm, respectively ([DAST] = 2 × 10−5 M). d), e) and f) represent the size distributions of a), b) and c). The scale bar represents 500 nm.](/image/article/2010/NR/b9nr00402e/b9nr00402e-f2.gif) | ||
| Fig. 2 SEM images of DAST NCs prepared without and with CSiO-G5. a), b) and c) CSiO-G5/DAST = 0, 4.1 and 8.8 with sizes of 212 ± 49 nm, 58 ± 7 nm and 54 ± 8 nm, respectively ([DAST] = 2 × 10−5 M). d), e) and f) represent the size distributions of a), b) and c). The scale bar represents 500 nm. | ||
The presence of CSiO-G5 in the reaction mixture causes not only a reduction in the size of DAST NCs but also plays a role in stabilizing their dispersion. By tracing the kinetic crystal growth process through UV-vis absorption spectra, the effect of CSiO-G5 on the precipitating process of DAST NCs was confirmed. The UV-vis absorption spectra shown in Fig. 3a demonstrate the kinetic process of DAST NCs prepared without the dendrimer. The broadening and red shift of the absorption bands during the DAST crystal growth process indicate the aggregation of molecules.13 The gradual decrease in the absorbance reveals the precipitation of DAST NCs. On the other hand, the absorption peaks do not show a subsequent red shift over time when CSiO-G5 is present in the preparation process of DAST NCs (Fig. 3b). This clearly proves the effect of the dendrimer in adjusting the crystal growth of DAST NCs during the precipitation process. Furthermore, the minor change in absorption proves that the dispersion of DAST NCs remains stable even after 5 h. It was found that changing the molar ratio of CSiO-G5/DAST does not cause a red shift of the absorption peaks. However, the decrease in the absorbance of DAST NCs at 475 nm becomes slower when the molar ratio of CSiO-G5/DAST increases from 4.1 to 8.8 (ESI, Fig. S1†). These results indicate that the addition of CSiO-G5 significantly alters the crystal growth process of DAST NCs, resulting in the generation and stabilization of regular nanosized DAST NCs.
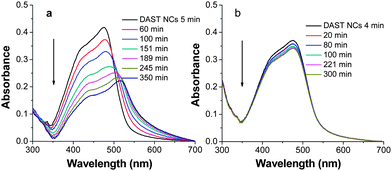 | ||
| Fig. 3 UV-Vis absorption spectra of DAST NCs (2 × 10−5 M in molecule) prepared a) without dendrimer and b) with CSiO-G5 (CSiO-G5/DAST = 8.8), respectively, at different evolution time. | ||
The fluorescence properties of DAST NCs are different from those of DAST molecular solution. Fig. 4a shows the fluorescence spectra of both a dispersion of NCs and a molecular solution of DAST with the same concentration. The DAST NCs dispersion displays a peak at 552 nm, which is blue-shifted compared to that of the DAST methanol solution at 587 nm. The fluorescence quantum yield is measured by using fluorescein in 0.1 N aqueous NaOH solution (Φ = 0.9) as a reference standard. When excited at 480 nm, the fluorescence quantum yield of the DAST NCs is 4.0%, which is about three times larger than that of 1.5% for the DAST NCs without CSiO-G5 and seven times larger than that of 0.6% for the DAST molecules in methanol. The enhanced fluorescence quantum yield of the DAST NCs most likely results from the dielectric constant altering because of changes in the solvent around the NCs, and the intra- and intermolecular interactions of the NCs. In addition, the DAST NCs show a longer lifetime than DAST molecules. Time resolved fluorescence measurements are carried out using a single photon fluorescence spectrometer from Edinburgh Instruments Co., UK. The decay data were collected at 545 nm with an excitation wavelength λex of 475 nm at room temperature. The lifetime of the DAST NCs is 3.95 ns, which is very close to 4.41 ns for the longer lifetime of the DAST NCs without CSiO-G5, and much longer than 88 ps for DAST solution (Fig. S3†).14 The radiative rate constant Kr was estimated as Kr = Φf/τ, and total nonradiative rate constant Knr as Knr = τ−1-Kr,15 where τ is the measured lifetime of the excited state and Φf is the fluorescence quantum yield. The ratios of Knr/Kr are around 30 and 166 for DAST NCs and molecules, respectively. This demonstrates that the radiative rate is significantly improved in DAST NCs compared with DAST molecules in solution. The formation of NCs restricts the rotation and vibration of DAST molecules, enhancing the fluorescence quantum yield and prolonging the excited state lifetime.
![a) Fluorescence spectra of DAST solution ([DAST] = 2 × 10−5 M) and CSiO-G5/DAST = 8.8 : 1 NCs (2 × 10−5 M), excited by 480 nm. b) Two-photon excited fluorescence (TPEF) spectra of DAST solution ([DAST] = 4 × 10−4 M) and CSiO-G5/DAST = 8.8 : 1 NCs (2 × 10−5 M), excited by 923 nm at 184 mW.](/image/article/2010/NR/b9nr00402e/b9nr00402e-f4.gif) | ||
Fig. 4 a) Fluorescence spectra of DAST solution ([DAST] = 2 × 10−5 M) and CSiO-G5/DAST = 8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NCs (2 × 10−5 M), excited by 480 nm. b) Two-photon excited fluorescence (TPEF) spectra of DAST solution ([DAST] = 4 × 10−4 M) and CSiO-G5/DAST = 8.8 1 NCs (2 × 10−5 M), excited by 480 nm. b) Two-photon excited fluorescence (TPEF) spectra of DAST solution ([DAST] = 4 × 10−4 M) and CSiO-G5/DAST = 8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NCs (2 × 10−5 M), excited by 923 nm at 184 mW. 1 NCs (2 × 10−5 M), excited by 923 nm at 184 mW. | ||
The nonlinear properties of DAST NCs were also investigated. A mode-locked Ti-sapphire laser was employed as the excitation source, of which the oscillating wavelength, pulse width and repetition rate are 923 nm, 80 fs and 82 MHz, respectively. There are two obvious peaks in the emission spectra of DAST NCs at a concentration of 2 × 10−5 M (Fig. 4b): a second harmonic generation (SHG) signal at 461.5 nm and up-converted fluorescence at 606 nm. Similar emission intensities are observed only when the concentration of DAST is increased to 4 × 10−4 M, which is in accordance with the broad two-photon excited fluorescence (TPEF) spectra reported for DAST.16 Referring to the TPEF method,17 the two-photon action cross section of the DAST NCs prepared with dendrimer is 22.3 GM at 923 nm, which is 23 times larger than that of the corresponding cross section of DAST molecules of 0.936 GM in methanol, using fluorescein as reference. However, the comparison with TPEF from nanocrystals prepared without CSiO-G5 cannot be performed due to the poor stability of the NC suspension. Because the two-photon absorption (TPA) cross section of charged push-pull molecules show little sensitivity to solvent polarity,18 we assume that the TPA cross section of DAST molecules in ether is the same as that in methanol. Thus, the TPA cross section of DAST NCs at 923 nm is 558 GM, with an enhancement factor of 3.58 compared to that of DAST molecules in solution of 156 GM. The SHG signal and improved up-converted emission of DAST NCs make them attractive for applications in lasing and bioimaging.
In summary, we successfully prepared a stable, size-controlled DAST NCs by a carbosiloxane dendrimer adjusting process. The size of the DAST NCs is successfully reduced to the nanometre scale. Compared to a molecular solution of DAST, the NCs exhibited a fluorescence quantum yield that was seven times larger and a much stronger up-converted fluorescence. The unique NLO properties of the DAST NCs provide potential for application in the fields of lasing and fluorescence probes.
Acknowledgements
We thanks Professor Masanori Ozaki at Osaka University for help with time-resolved fluorescence measurements. This work was financially supported by the National Basic Research Program (2010CB93 4103), Natural Science Foundation of China (NSFC, No. 50773091, No. 20702056), and the Core Research for Evolution Science and Technology (CREST) project of the Japan, Science and Technology Corporation (JST).References
- Y. Zhao, H. Fu, F. Hu, A. Peng, W. Yang and J. Yao, Adv. Mater., 2008, 20, 79 CrossRef.
- H. Oikawa, S. Fujita, H. Kasai, S. Okada, S. K. Tripathy and H. Nakanishi, Colloids Surf., A, 2000, 169, 251 CrossRef CAS.
- (a) S. R. Marder, J. W. Perry and C. P. Yakymyshyn, Chem. Mater., 1994, 6, 1137 CrossRef CAS; (b) X.-M. Duan, S. Okada, H. Oikawa, H. Matsuda and H. Nakanishi, Mol. Cryst. Liq. Cryst., 1995, 267, 89 CrossRef CAS; (c) X.-M. Duan, H. Konami, S. Okada, H. Oikawa, H. Matsuda and H. Nakanishi, J. Phys. Chem., 1996, 100, 17780 CrossRef CAS.
- H. Adachi, Y. Takahashi, J. Yabuzaki, Y. Mori and T. Sasaki, J. Cryst. Growth, 1999, 198–199, 568 CrossRef.
- Y. Kaneko, S. Shimada, T. Fukuda, T. Kimura, H. Yokoi, H. Matsuda, T. Onodera, H. Kasai, S. Okada, H. Oikawa and H. Nakanishi, Adv. Mater., 2005, 17, 160 CrossRef CAS.
- B. Ruiz, M. Jazbinsek and P. Günter, Cryst. Growth Des., 2008, 8, 4173 CrossRef CAS.
- H. Frey and C. Schlenk, Top. Curr. Chem., 2000, 210, 69 CAS.
- F. Bertorelle, D. Lavabre and S. Fery-Forgues, J. Am. Chem. Soc., 2003, 125, 6244 CrossRef CAS.
- (a) M.-L. Zheng, W.-Q. Chen, C.-F. Li, X.-Z. Dong and X.-M. Duan, ChemPhysChem, 2007, 8, 810 CrossRef CAS; (b) M.-L. Zheng, W.-Q. Chen and X.-M. Duan, J. Phys. Chem. A, 2008, 112, 6864 CrossRef CAS.
- H. Nakanishi, H. Matsuda, S. Okada and M. Kato, Adv. Mater., 1989, 1, 97 CrossRef.
- M.-L. Zheng, Q.-L. Tang, W.-Q. Chen and X.-M. Duan, J. Nanosci. Nanotechnol., 2009, 9, 1291 CrossRef CAS.
- H. Kasai, H. S. Nalwa, H. Oikawa, S. Okada, H. Matsuda, N. Minami, A. Kakuta, K. Ono, A. Mukoh and H. Nakanishi, Jpn. J. Appl. Phys., 1992, 31, L1132 CAS.
- S. Fujita, H. Kasai, S. Okada, H. Oikawa, T. Fukuda, H. Matsuda, S. K. Tripathy and H. Nakanishi, Jpn. J. Appl. Phys., 1999, 38, L659 CrossRef CAS.
- Time resolved fluorescence measurement was carried out using a streak scope camera (Hamamatsu, C4334) with the excitation of femtosecond laser pulse at the wavelength of 400 nm. The time resolution of the system was limited by the time resolution of the streak camera of about 10ps.
- S. Li, L. He, F. Xiong, Y. Li and G. Yang, J. Phys. Chem. B, 2004, 108, 10887 CrossRef CAS.
- K. Kumar, R. N. Rai and S. B. Rai, Appl. Phys. B: Lasers Opt., 2009, 96, 85 CrossRef CAS.
- (a) G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245 CrossRef CAS; (b) C. Xu and W. W. Webb, J. Opt. Soc. Am. B, 1996, 13, 481 Search PubMed.
- D. Laage, W. H. Thompson, M. B. Desce and J. T. Hynes, J. Phys. Chem. A, 2003, 107, 6032 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the experimental procedure and spectroscopic characterization. See DOI: 10.1039/b9nr00402e |
| This journal is © The Royal Society of Chemistry 2010 |
